IroT/mavN, a new iron-regulated gene involved in Legionella pneumophila virulence against amoebae and macrophages
- PMID: 25141909
- PMCID: PMC4672373
- DOI: 10.1111/1462-2920.12604
IroT/mavN, a new iron-regulated gene involved in Legionella pneumophila virulence against amoebae and macrophages
Abstract
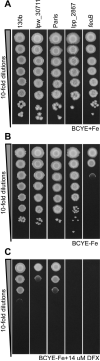
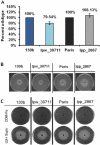
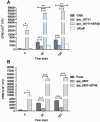
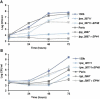
Legionella pneumophila is a pathogenic bacterium commonly found in water. Eventually, it could be transmitted to humans via inhalation of contaminated aerosols. Iron is known as a key requirement for the growth of L. pneumophila in the environment and within its hosts. Many studies were performed to understand iron utilization by L. pneumophila but no global approaches were conducted. In this study, transcriptomic analyses were performed, comparing gene expression in L. pneumophila in standard versus iron restricted conditions. Among the regulated genes, a newly described one, lpp_2867, was highly induced in iron-restricted conditions. Mutants lacking this gene in L. pneumophila were not affected in siderophore synthesis or utilization. On the contrary, they were defective for growth on iron-depleted solid media and for ferrous iron uptake. A sequence analysis predicts that Lpp_2867 is a membrane protein, suggesting that it is involved in ferrous iron transport. We thus named it IroT, for iron transporter. Infection assays showed that the mutants are highly impaired in intracellular growth within their environmental host Acanthamoeba castellanii and human macrophages. Taken together, our results show that IroT is involved, directly or indirectly, in ferrous iron transport and is a key virulence factor for L. pneumophila.
© 2014 Society for Applied Microbiology and John Wiley & Sons Ltd.
Figures

References
-
- Aisen P. The binding and release of iron by transferrin. Birth Defects Orig Artic Ser. 1976;12:81–95. - PubMed
-
- Andrews SC. Making DNA without iron - induction of a manganese-dependent ribonucleotide reductase in response to iron starvation. Mol Microbiol. 2011;80:286–289. - PubMed
-
- Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

