Feasibility of amylin imaging in pancreatic islets with β-amyloid imaging probes
- PMID: 25142178
- PMCID: PMC4139942
- DOI: 10.1038/srep06155
Feasibility of amylin imaging in pancreatic islets with β-amyloid imaging probes
Abstract
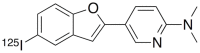
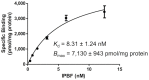
Islet amyloid deposition composed of amylin aggregates is regarded as one of the hallmarks of type 2 diabetes mellitus (T2DM). For the diagnosis of T2DM, several nuclear medical imaging probes have been developed. However, there have been no reports regarding the development of imaging probes targeting amylin. In this report, we investigated the feasibility of amylin imaging using [(125)I]IPBF as one of the model compounds of β-amyloid (Aβ) imaging probes. In in vitro experiments, [(125)I]IPBF exhibited high binding affinity for amylin aggregates (Kd = 8.31 nM). Moreover, autoradiographic images showed that [(125)I]IPBF specifically bound to islet amyloid composed of amylin. These results suggest the potential application of Aβ imaging probes to amylin imaging. In addition, [(125)I]IPBF is one of the promising lead compounds for amylin imaging, and further structural optimization based on [(125)I]IPBF may lead to useful tracers for the in vivo imaging of islet amyloids in the pancreas.
Figures
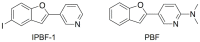
Similar articles
-
Development of Novel PET Imaging Probes for Detection of Amylin Aggregates in the Pancreas.Mol Pharm. 2020 Apr 6;17(4):1293-1299. doi: 10.1021/acs.molpharmaceut.9b01309. Epub 2020 Mar 23. Mol Pharm. 2020. PMID: 32202808
-
Characterization of Novel 18F-Labeled Phenoxymethylpyridine Derivatives as Amylin Imaging Probes.Mol Pharm. 2018 Dec 3;15(12):5574-5584. doi: 10.1021/acs.molpharmaceut.8b00756. Epub 2018 Nov 8. Mol Pharm. 2018. PMID: 30407835
-
Development of (99m)Tc-Labeled Pyridyl Benzofuran Derivatives To Detect Pancreatic Amylin in Islet Amyloid Model Mice.Bioconjug Chem. 2016 Jun 15;27(6):1532-9. doi: 10.1021/acs.bioconjchem.6b00174. Epub 2016 May 24. Bioconjug Chem. 2016. PMID: 27219875
-
Amylin uncovered: a review on the polypeptide responsible for type II diabetes.Biomed Res Int. 2013;2013:826706. doi: 10.1155/2013/826706. Epub 2013 Mar 31. Biomed Res Int. 2013. PMID: 23607096 Free PMC article. Review.
-
Islet amyloid polypeptide, islet amyloid, and diabetes mellitus.Physiol Rev. 2011 Jul;91(3):795-826. doi: 10.1152/physrev.00042.2009. Physiol Rev. 2011. PMID: 21742788 Review.
Cited by
-
The β Cell in Diabetes: Integrating Biomarkers With Functional Measures.Endocr Rev. 2021 Sep 28;42(5):528-583. doi: 10.1210/endrev/bnab021. Endocr Rev. 2021. PMID: 34180979 Free PMC article. Review.
-
Use of the PET ligand florbetapir for in vivo imaging of pancreatic islet amyloid deposits in hIAPP transgenic mice.Diabetologia. 2018 Oct;61(10):2215-2224. doi: 10.1007/s00125-018-4695-y. Epub 2018 Jul 25. Diabetologia. 2018. PMID: 30046852 Free PMC article.
-
An Iridium(III) Complex as a Photoactivatable Tool for Oxidation of Amyloidogenic Peptides with Subsequent Modulation of Peptide Aggregation.Chemistry. 2017 Jan 31;23(7):1645-1653. doi: 10.1002/chem.201604751. Epub 2017 Jan 3. Chemistry. 2017. PMID: 27862428 Free PMC article.
-
Genome-wide interaction study of brain beta-amyloid burden and cognitive impairment in Alzheimer's disease.Mol Psychiatry. 2017 Feb;22(2):287-295. doi: 10.1038/mp.2016.35. Epub 2016 Mar 29. Mol Psychiatry. 2017. PMID: 27021820 Free PMC article.
References
-
- Shaw J. E., Sicree R. A. & Zimmet P. Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14 (2010). - PubMed
-
- Izucchi S. E. Clinical practice. Diagnosis of diabetes. N. Engl. J. Med. 367, 542–550 (2012). - PubMed
-
- Meier J. J. et al. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia, 55, 1346–1354 (2012). - PubMed
-
- Rocken C., Linke R. P. & Saeger W. Immunohistology of islet amyloid polypeptide in diabetes mellitus: semi-quantitative studies in a post-mortem series. Virchows Arch. A: Pathol. Anat. Histopathol. 421, 339–344 (1992). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

