Copackaging of multiple adeno-associated viral vectors in a single production step
- PMID: 25143183
- PMCID: PMC4346231
- DOI: 10.1089/hgtb.2014.055
Copackaging of multiple adeno-associated viral vectors in a single production step
Abstract
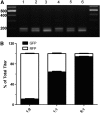
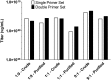
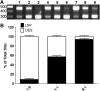
Limiting factors in large preclinical and clinical studies utilizing adeno-associated virus (AAV) for gene therapy are focused on the restrictive packaging capacity, the overall yields, and the versatility of the production methods for single AAV vector production. Furthermore, applications where multiple vectors are needed to provide long expression cassettes, whether because of long cDNA sequences or the need of different regulatory elements, require that each vector be packaged and characterized separately, directly affecting labor and cost associated with such manufacturing strategies. To overcome these limitations, we propose a novel method of vector production that allows for the packaging of multiple expression cassettes in a single transfection step. Here we combined two expression cassettes in predetermined ratios before transfection and empirically demonstrate that the output vector recapitulates the predicted ratios. Titration by quantitative polymerase chain reaction of AAV vector genome copies using shared or unique genetic elements allowed for delineation of the individual vector contribution to the total preparation that showed the predicted differential packaging outcomes. By copackaging green fluorescent protein (GFP) and mCherry constructs, we demonstrate that both vector genome and infectious titers reiterated the ratios utilized to produce the constructs by transfection. Copackaged therapeutic constructs that only differ in transcriptional elements produced a heterogeneous vector population of both constructs in the predefined ratios. This study shows feasibility and reproducibility of a method that allows for two constructs, differing in either transgene or transcription elements, to be efficiently copackaged and characterized simultaneously, reducing cost of manufacturing and release testing.
Figures

References
-
- Boutin S., Monteilhet V., Veron P., et al. (2010). Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 21, 704–712 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

