Quantitative analysis and modeling of katanin function in flagellar length control
- PMID: 25143397
- PMCID: PMC4230626
- DOI: 10.1091/mbc.E14-06-1116
Quantitative analysis and modeling of katanin function in flagellar length control
Abstract
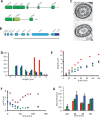
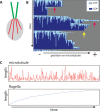
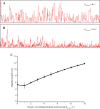
Flagellar length control in Chlamydomonas reinhardtii provides a simple model system in which to investigate the general question of how cells regulate organelle size. Previous work demonstrated that Chlamydomonas cytoplasm contains a pool of flagellar precursor proteins sufficient to assemble a half-length flagellum and that assembly of full-length flagella requires synthesis of additional precursors to augment the preexisting pool. The regulatory systems that control the synthesis and regeneration of this pool are not known, although transcriptional regulation clearly plays a role. We used quantitative analysis of length distributions to identify candidate genes controlling pool regeneration and found that a mutation in the p80 regulatory subunit of katanin, encoded by the PF15 gene in Chlamydomonas, alters flagellar length by changing the kinetics of precursor pool utilization. This finding suggests a model in which flagella compete with cytoplasmic microtubules for a fixed pool of tubulin, with katanin-mediated severing allowing easier access to this pool during flagellar assembly. We tested this model using a stochastic simulation that confirms that cytoplasmic microtubules can compete with flagella for a limited tubulin pool, showing that alteration of cytoplasmic microtubule severing could be sufficient to explain the effect of the pf15 mutations on flagellar length.
© 2014 Kannegaard et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures



References
-
- Blaineau C, Tessier M, Dubessay P, Tasse L, Crobu L, Pages M, Bastien P. A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr Biol. 2007;17:778–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

