Loss of γ-cytoplasmic actin triggers myofibroblast transition of human epithelial cells
- PMID: 25143399
- PMCID: PMC4196865
- DOI: 10.1091/mbc.E14-03-0815
Loss of γ-cytoplasmic actin triggers myofibroblast transition of human epithelial cells
Abstract
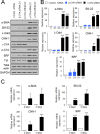
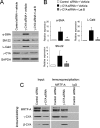
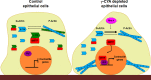
Transdifferentiation of epithelial cells into mesenchymal cells and myofibroblasts plays an important role in tumor progression and tissue fibrosis. Such epithelial plasticity is accompanied by dramatic reorganizations of the actin cytoskeleton, although mechanisms underlying cytoskeletal effects on epithelial transdifferentiation remain poorly understood. In the present study, we observed that selective siRNA-mediated knockdown of γ-cytoplasmic actin (γ-CYA), but not β-cytoplasmic actin, induced epithelial-to-myofibroblast transition (EMyT) of different epithelial cells. The EMyT manifested by increased expression of α-smooth muscle actin and other contractile proteins, along with inhibition of genes responsible for cell proliferation. Induction of EMyT in γ-CYA-depleted cells depended on activation of serum response factor and its cofactors, myocardial-related transcriptional factors A and B. Loss of γ-CYA stimulated formin-mediated actin polymerization and activation of Rho GTPase, which appear to be essential for EMyT induction. Our findings demonstrate a previously unanticipated, unique role of γ-CYA in regulating epithelial phenotype and suppression of EMyT that may be essential for cell differentiation and tissue fibrosis.
© 2014 Lechuga, Baranwal, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures







Similar articles
-
Induction of Fibroblast-to-Myofibroblast Differentiation by Changing Cytoplasmic Actin Ratio.Biochemistry (Mosc). 2025 Feb;90(2):289-298. doi: 10.1134/S000629792460412X. Biochemistry (Mosc). 2025. PMID: 40254406
-
β-catenin and Smad3 regulate the activity and stability of myocardin-related transcription factor during epithelial-myofibroblast transition.Mol Biol Cell. 2011 Dec;22(23):4472-85. doi: 10.1091/mbc.E11-04-0335. Epub 2011 Sep 30. Mol Biol Cell. 2011. PMID: 21965288 Free PMC article.
-
Nonredundant roles of cytoplasmic β- and γ-actin isoforms in regulation of epithelial apical junctions.Mol Biol Cell. 2012 Sep;23(18):3542-53. doi: 10.1091/mbc.E12-02-0162. Epub 2012 Aug 1. Mol Biol Cell. 2012. PMID: 22855531 Free PMC article.
-
The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation.J Cardiovasc Transl Res. 2012 Dec;5(6):794-804. doi: 10.1007/s12265-012-9397-0. Epub 2012 Aug 17. J Cardiovasc Transl Res. 2012. PMID: 22898751 Review.
-
Smaddening complexity: the role of Smad3 in epithelial-myofibroblast transition.Cells Tissues Organs. 2011;193(1-2):41-52. doi: 10.1159/000320180. Epub 2010 Nov 3. Cells Tissues Organs. 2011. PMID: 21051861 Review.
Cited by
-
Extracellular Matrix in Regulation of Contractile System in Cardiomyocytes.Int J Mol Sci. 2019 Oct 11;20(20):5054. doi: 10.3390/ijms20205054. Int J Mol Sci. 2019. PMID: 31614676 Free PMC article. Review.
-
SMIFH2 has effects on Formins and p53 that perturb the cell cytoskeleton.Sci Rep. 2015 Apr 30;5:9802. doi: 10.1038/srep09802. Sci Rep. 2015. PMID: 25925024 Free PMC article.
-
Diversity from similarity: cellular strategies for assigning particular identities to actin filaments and networks.Open Biol. 2020 Sep;10(9):200157. doi: 10.1098/rsob.200157. Epub 2020 Sep 2. Open Biol. 2020. PMID: 32873155 Free PMC article.
-
A feedback circuitry involving γ-actin, β-actin and nonmuscle myosin-2 A controls tight junction and apical cortex mechanics.Nat Commun. 2025 Mar 13;16(1):2514. doi: 10.1038/s41467-025-57428-y. Nat Commun. 2025. PMID: 40082413 Free PMC article.
-
Expression of α-Smooth Muscle Actin Determines the Fate of Mesenchymal Stromal Cells.Stem Cell Reports. 2015 Jun 9;4(6):1016-30. doi: 10.1016/j.stemcr.2015.05.004. Epub 2015 May 28. Stem Cell Reports. 2015. PMID: 26028530 Free PMC article.
References
-
- Baarlink C, Wang H, Grosse R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science. 2013;340:864–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

