Systematic lipidomic analysis of yeast protein kinase and phosphatase mutants reveals novel insights into regulation of lipid homeostasis
- PMID: 25143408
- PMCID: PMC4196872
- DOI: 10.1091/mbc.E14-03-0851
Systematic lipidomic analysis of yeast protein kinase and phosphatase mutants reveals novel insights into regulation of lipid homeostasis
Abstract
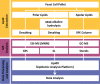
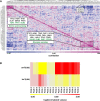
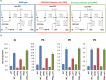
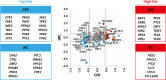
The regulatory pathways required to maintain eukaryotic lipid homeostasis are largely unknown. We developed a systematic approach to uncover new players in the regulation of lipid homeostasis. Through an unbiased mass spectrometry-based lipidomic screening, we quantified hundreds of lipid species, including glycerophospholipids, sphingolipids, and sterols, from a collection of 129 mutants in protein kinase and phosphatase genes of Saccharomyces cerevisiae. Our approach successfully identified known kinases involved in lipid homeostasis and uncovered new ones. By clustering analysis, we found connections between nutrient-sensing pathways and regulation of glycerophospholipids. Deletion of members of glucose- and nitrogen-sensing pathways showed reciprocal changes in glycerophospholipid acyl chain lengths. We also found several new candidates for the regulation of sphingolipid homeostasis, including a connection between inositol pyrophosphate metabolism and complex sphingolipid homeostasis through transcriptional regulation of AUR1 and SUR1. This robust, systematic lipidomic approach constitutes a rich, new source of biological information and can be used to identify novel gene associations and function.
© 2014 da Silveira dos Santos et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


References
-
- Barbosa AD, Osório H, Sims KJ, Almeida T, Alves M, Bielawski J, Amorim MA, Moradas-Ferreira P, Hannun YA, Costa V. Role for Sit4p-dependent mitochondrial dysfunction in mediating the shortened chronological lifespan and oxidative stress sensitivity of Isc1p-deficient cells. Mol Microbiol. 2011;81:515–527. - PMC - PubMed
-
- Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, Walther TC, Loewith R. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat Cell Biol. 2012;14:542–547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

