Theta and high-frequency activity mark spontaneous recall of episodic memories
- PMID: 25143616
- PMCID: PMC4138344
- DOI: 10.1523/JNEUROSCI.2654-13.2014
Theta and high-frequency activity mark spontaneous recall of episodic memories
Abstract
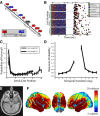
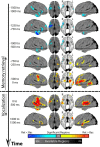
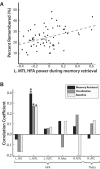
Humans possess the remarkable ability to search their memory, allowing specific past episodes to be re-experienced spontaneously. Here, we administered a free recall test to 114 neurosurgical patients and used intracranial theta and high-frequency activity (HFA) to identify the spatiotemporal pattern of neural activity underlying spontaneous episodic retrieval. We found that retrieval evolved in three electrophysiological stages composed of: (1) early theta oscillations in the right temporal cortex, (2) increased HFA in the left hemisphere including the medial temporal lobe (MTL), left inferior frontal gyrus, as well as the ventrolateral temporal cortex, and (3) motor/language activation during vocalization of the retrieved item. Of these responses, increased HFA in the left MTL predicted recall performance. These results suggest that spontaneous recall of verbal episodic memories involves a spatiotemporal pattern of spectral changes across the brain; however, high-frequency activity in the left MTL represents a final common pathway of episodic retrieval.
Keywords: ECoG; episodic retrieval; high-frequency activity; memory; theta.
Copyright © 2014 the authors 0270-6474/14/3411355-11$15.00/0.
Figures




References
-
- Addison PS. The illustrated wavelet transform handbook: introductory theory and applications in science, engineering, medicine and finance. Bristol: Institute of Physics; 2002.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
