Amyloid-β-induced action potential desynchronization and degradation of hippocampal gamma oscillations is prevented by interference with peptide conformation change and aggregation
- PMID: 25143621
- PMCID: PMC6615507
- DOI: 10.1523/JNEUROSCI.1195-14.2014
Amyloid-β-induced action potential desynchronization and degradation of hippocampal gamma oscillations is prevented by interference with peptide conformation change and aggregation
Abstract
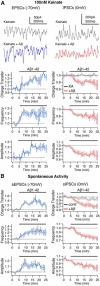
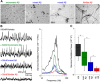
The amyloid-β hypothesis of Alzheimer's Disease (AD) focuses on accumulation of amyloid-β peptide (Aβ) as the main culprit for the myriad physiological changes seen during development and progression of AD including desynchronization of neuronal action potentials, consequent development of aberrant brain rhythms relevant for cognition, and final emergence of cognitive deficits. The aim of this study was to elucidate the cellular and synaptic mechanisms underlying the Aβ-induced degradation of gamma oscillations in AD, to identify aggregation state(s) of Aβ that mediate the peptides neurotoxicity, and to test ways to prevent the neurotoxic Aβ effect. We show that Aβ(1-42) in physiological concentrations acutely degrades mouse hippocampal gamma oscillations in a concentration- and time-dependent manner. The underlying cause is an Aβ-induced desynchronization of action potential generation in pyramidal cells and a shift of the excitatory/inhibitory equilibrium in the hippocampal network. Using purified preparations containing different aggregation states of Aβ, as well as a designed ligand and a BRICHOS chaperone domain, we provide evidence that the severity of Aβ neurotoxicity increases with increasing concentration of fibrillar over monomeric Aβ forms, and that Aβ-induced degradation of gamma oscillations and excitatory/inhibitory equilibrium is prevented by compounds that interfere with Aβ aggregation. Our study provides correlative evidence for a link between Aβ-induced effects on synaptic currents and AD-relevant neuronal network oscillations, identifies the responsible aggregation state of Aβ and proofs that strategies preventing peptide aggregation are able to prevent the deleterious action of Aβ on the excitatory/inhibitory equilibrium and on the gamma rhythm.
Keywords: Alzheimer's disease; BRICHOS domain; amyloid-β peptide; gamma oscillations; hippocampus; neuronal synchronization.
Copyright © 2014 the authors 0270-6474/14/3411416-10$15.00/0.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
