Regulation of blood flow in the retinal trilaminar vascular network
- PMID: 25143628
- PMCID: PMC4138352
- DOI: 10.1523/JNEUROSCI.1971-14.2014
Regulation of blood flow in the retinal trilaminar vascular network
Abstract
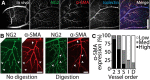
Light stimulation evokes neuronal activity in the retina, resulting in the dilation of retinal blood vessels and increased blood flow. This response, named functional hyperemia, brings oxygen and nutrients to active neurons. However, it remains unclear which vessels mediate functional hyperemia. We have characterized blood flow regulation in the rat retina in vivo by measuring changes in retinal vessel diameter and red blood cell (RBC) flux evoked by a flickering light stimulus. We found that, in first- and second-order arterioles, flicker evoked large (7.5 and 5.0%), rapid (0.73 and 0.70 s), and consistent dilations. Flicker-evoked dilations in capillaries were smaller (2.0%) and tended to have a slower onset (0.97 s), whereas dilations in venules were smaller (1.0%) and slower (1.06 s) still. The proximity of pericyte somata did not predict capillary dilation amplitude. Expression of the contractile protein α-smooth muscle actin was high in arterioles and low in capillaries. Unexpectedly, we found that blood flow in the three vascular layers was differentially regulated. Flicker stimulation evoked far larger dilations and RBC flux increases in the intermediate layer capillaries than in the superficial and deep layer capillaries (2.6 vs 0.9 and 0.7% dilation; 25.7 vs 0.8 and 11.3% RBC flux increase). These results indicate that functional hyperemia in the retina is driven primarily by active dilation of arterioles. The dilation of intermediate layer capillaries is likely mediated by active mechanisms as well. The physiological consequences of differential regulation in the three vascular layers are discussed.
Keywords: blood flow; capillary; functional hyperemia; pericyte; rat; retina.
Copyright © 2014 the authors 0270-6474/14/3411504-10$15.00/0.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
