Independent assessment of candidate HIV incidence assays on specimens in the CEPHIA repository
- PMID: 25144218
- PMCID: PMC4210690
- DOI: 10.1097/QAD.0000000000000429
Independent assessment of candidate HIV incidence assays on specimens in the CEPHIA repository
Abstract
Objective: Cross-sectional HIV incidence surveillance, using assays that distinguish 'recent' from 'nonrecent' infections, has been hampered by inadequate performance and characterization of incidence assays. In this study, the Consortium for the Evaluation and Performance of HIV Incidence Assays presents results of the first independent evaluation of five incidence assays (BED, Limiting Antigen Avidity, Less-sensitive Vitros, Vitros Avidity and BioRad Avidity).
Design: A large repository of diverse specimens from HIV-positive patients was established, multiple assays were run on 2500 selected specimens, and data were analyzed to estimate assay characteristics relevant for incidence surveillance.
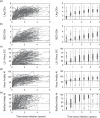
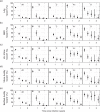
Methods: The mean duration of recent infection (MDRI, average time 'recent' while infected for less than some time cut-off T) was estimated from longitudinal data on seroconverters by regression. The false-recent rate (FRR, probability of testing 'recent' when infected for longer than T) was explored by measuring the proportions of 'recent' results in various subsets of patients.
Results: Assays continue to fail to attain the simultaneously large MDRI and small FRR demanded by existing performance guidelines. All assays produce high FRRs amongst virally suppressed patients (>40%), including elite controllers and treated patients.
Conclusions: Results from this first independent evaluation provide valuable information about the current performance of assays, and suggest the need for further optimization. Variation of 'recent'/'nonrecent' thresholds and the use of multiple antibody-maturation assays, as well as other biomarkers, can now be explored, using the rich data generated by the Consortium for the Evaluation and Performance of HIV Incidence Assays. Consistently high FRRs amongst those virally suppressed suggest that viral load will be a particularly valuable supplementary marker.
2014 Wolters Kluwer Health | Lippincott Williams & Wilkins
Figures

References
-
- Brookmeyer R, Quinn TC. Estimation of current human immunodeficiency virus incidence rates from a cross-sectional survey using early diagnostic tests. Am J Epidemiol 1995; 141:166–172 - PubMed
-
- UNAIDS/WHO working group on global HIV/AIDS and STI surveillance. When and how to use assays for recent infection to estimate HIV incidence at a population level. 2011. www.who.int/diagnostics_laboratory/hiv_incidence_may13_final.pdf [Accessed 29 March 2014]
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

