Nifedipine plus candesartan combination increases blood pressure control regardless of race and improves the side effect profile: DISTINCT randomized trial results
- PMID: 25144296
- PMCID: PMC4227617
- DOI: 10.1097/HJH.0000000000000331
Nifedipine plus candesartan combination increases blood pressure control regardless of race and improves the side effect profile: DISTINCT randomized trial results
Abstract
Objectives: DISTINCT (reDefining Intervention with Studies Testing Innovative Nifedipine GITS - Candesartan Therapy) aimed to determine the dose-response and tolerability of nifedipine GITS and/or candesartan cilexetil therapy in participants with hypertension.
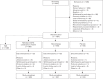
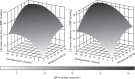
Methods: In this 8-week, multinational, multicentre, randomized, double-blind, placebo-controlled study, adults with mean seated DBP of at least 95 to less than 110 mmHg received combination or monotherapy with nifedipine GITS (N) 20, 30 or 60 mg and candesartan cilexetil (C) 4, 8, 16 or 32 mg, or placebo. The primary endpoint, change in DBP from baseline to Week 8, was analysed using the response surface model (RSM); this analysis was repeated for mean seated SBP.
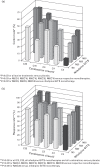
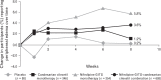
Results: Overall, 1381 participants (mean baseline SBP/DBP: 156.5/99.6 mmHg) were randomized. Both N and C contributed independently to SBP/DBP reductions [P < 0.0001 (RSM)]. A positive dose-response was observed, with all combinations providing statistically better blood pressure (BP) reductions from baseline versus respective monotherapies (P < 0.05) and N60C32 achieving the greatest reduction [-23.8/-16.5 mmHg; P < 0.01 versus placebo (-5.3/-6.7 mmHg) and component monotherapies]. Even very low-dose (N20 and C4) therapy provided significant BP-lowering, and combination therapy was similarly effective in different racial groups. N/C combination demonstrated a lower incidence of vasodilatory adverse events than N monotherapy (18.3 versus 23.6%), including headache (5.5 versus 11.0%; P = 0.003, chi-square test) and peripheral oedema over time (3.6 versus 5.8%; n.s.).
Conclusion: N/C combination was effective in participants with hypertension and showed an improved side effect profile compared with N monotherapy.
Trial registration: ClinicalTrials.gov NCT01303783.
Figures


References
-
- Weir MR, Zappe D, Orloski LA, Sowers JR. How early should blood pressure control be achieved for optimal cardiovascular outcomes? J Hum Hypertens 2011; 25:211–217. - PubMed
-
- Kjeldsen SE, Messerli FH, Chiang CE, Meredith PA, Liu L. Are fixed-dose combination antihypertensives suitable as first-line therapy? Curr Med Res Opin 2012; 28:1685–1697. - PubMed
-
- Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med 2011; 124:128–135. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

