Effects of acute versus post-acute systemic delivery of neural progenitor cells on neurological recovery and brain remodeling after focal cerebral ischemia in mice
- PMID: 25144721
- PMCID: PMC4454329
- DOI: 10.1038/cddis.2014.359
Effects of acute versus post-acute systemic delivery of neural progenitor cells on neurological recovery and brain remodeling after focal cerebral ischemia in mice
Abstract
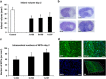
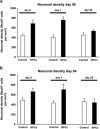
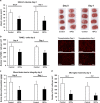
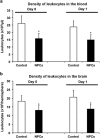
Intravenous transplantation of neural progenitor cells (NPCs) induces functional recovery after stroke, albeit grafted cells are not integrated into residing neural networks. However, a systematic analysis of intravenous NPC delivery at acute and post-acute time points and their long-term consequences does not exist. Male C57BL6 mice were exposed to cerebral ischemia, and NPCs were intravenously grafted on day 0, on day 1 or on day 28. Animals were allowed to survive for up to 84 days. Mice and tissues were used for immunohistochemical analysis, flow cytometry, ELISA and behavioral tests. Density of grafted NPCs within the ischemic hemisphere was increased when cells were transplanted on day 28 as compared with transplantation on days 0 or 1. Likewise, transplantation on day 28 yielded enhanced neuronal differentiation rates of grafted cells. Post-ischemic brain injury, however, was only reduced when NPCs were grafted at acute time points. On the contrary, reduced post-ischemic functional deficits due to NPC delivery were independent of transplantation paradigms. NPC-induced neuroprotection after acute cell delivery was due to stabilization of the blood-brain barrier (BBB), reduction in microglial activation and modulation of both peripheral and central immune responses. On the other hand, post-acute NPC transplantation stimulated post-ischemic regeneration via enhanced angioneurogenesis and increased axonal plasticity. Acute NPC delivery yields long-term neuroprotection via enhanced BBB integrity and modulation of post-ischemic immune responses, whereas post-acute NPC delivery increases post-ischemic angioneurogenesis and axonal plasticity. Post-ischemic functional recovery, however, is independent of NPC delivery timing, which offers a broad therapeutic time window for stroke treatment.
Figures


References
-
- Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265:73–77. - PubMed
-
- Doeppner TR, Ewert TA, Tonges L, Herz J, Zechariah A, Elali A, et al. Transduction of neural precursor cells with TAT-heat shock protein 70 chaperone: therapeutic potential against ischemic stroke after intrastriatal and systemic transplantation. Stem cells. 2012;30:1297–1310. - PubMed
-
- Zheng W, Honmou O, Miyata K, Harada K, Suzuki J, Liu H, et al. Therapeutic benefits of human mesenchymal stem cells derived from bone marrow after global cerebral ischemia. Brain Res. 2010;1310:8–16. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

