Synthesis of nucleoside phosphate and phosphonate prodrugs
- PMID: 25144792
- PMCID: PMC4173794
- DOI: 10.1021/cr5002035
Synthesis of nucleoside phosphate and phosphonate prodrugs
Figures
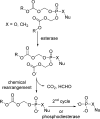
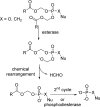
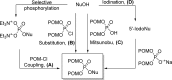
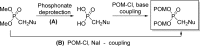


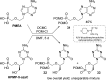
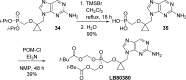
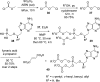
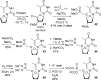
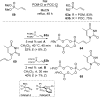
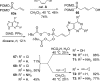
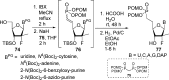
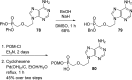
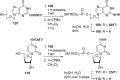
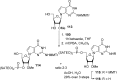

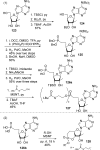

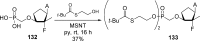


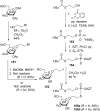
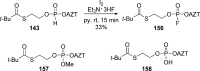
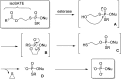
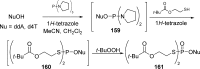
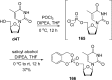
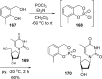
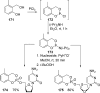
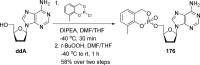

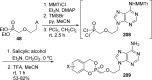
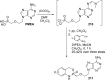
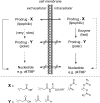
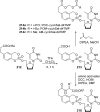
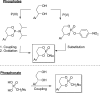
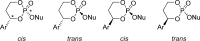
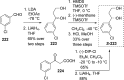
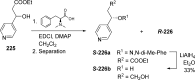
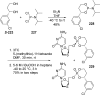
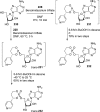
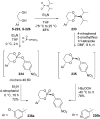

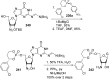

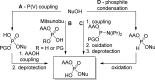
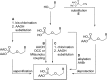
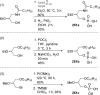
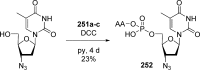

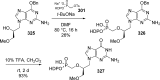
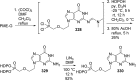

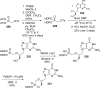
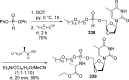
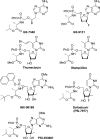
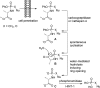
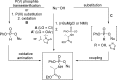
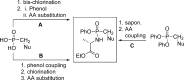
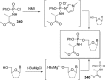
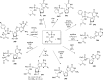
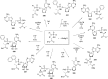
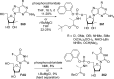
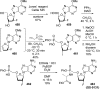
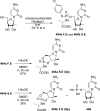
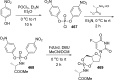
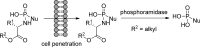
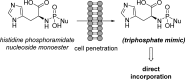
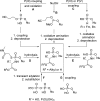

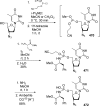
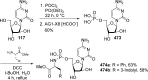
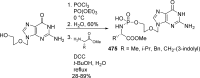
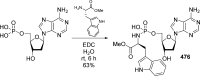
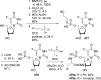
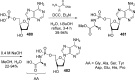
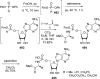
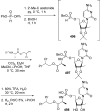
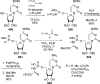
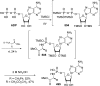
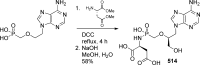
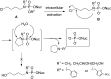
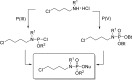
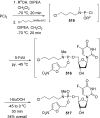
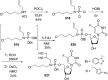

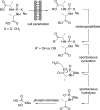
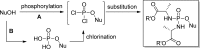
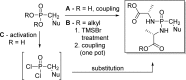
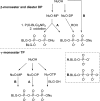
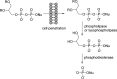
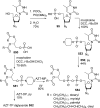
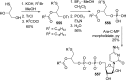
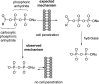

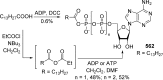
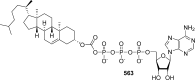
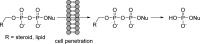
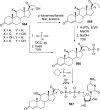
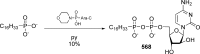
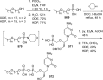
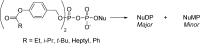
References
-
- Van Rompay A. R.; Johansson M.; Karlsson A. Pharmacol. Ther. 2000, 87, 189. - PubMed
-
- Lin C.-C.; Yeh L.-T.; Vitarella D.; Hong Z.; Erion M. D. Antiviral Chem. Chemother. 2004, 15, 307. - PubMed
- Erion M. D.; van Poelje P. D.; MacKenna D. A.; Colby T. J.; Montag A. C.; Fujitaki J. M.; Linemeyer D. L.; Bullough D. A. J. Pharmacol. Exp. Ther. 2005, 312, 554. - PubMed
- Erion M. D.; Bullough D. A.; Lin C.-C.; Hong Z. Curr. Opin. Invest. Drugs 2006, 7, 109. - PubMed
- Reddy K. R.; Matelich M. C.; Ugarkar B. G.; Gomez-Galeno J. E.; DaRe J.; Ollis K.; Sun Z.; Craigo W.; Colby T. J.; Fujitaki J. M.; Boyer S. B.; van Poelje P. D.; Erion M. D. J. Med. Chem. 2008, 51, 666. - PubMed
-
- Eisenberg E. J.; He G. X.; Lee W. A. Nucleosides, Nucleotides Nucleic Acids 2001, 20, 1091. - PubMed
-
- Naesens L.; Bischofberger N.; Augustijns P.; Annaert P.; Van den Mooter G.; Arimilli M. N.; Kim C. U.; De Clercq E. Antimicrob. Agents Chemother. 1998, 42, 1568. - PMC - PubMed
- Robbins B. L.; Srinivas R. V.; Kim C.; Bischofberger N.; Fridland A. Antimicrob. Agents Chemother. 1998, 42, 612. - PMC - PubMed
- Schooley R. T.; Ruane P.; Myers R. A.; Beall G.; Lampiris H.; Berger D.; Chen S.-S.; Miller M. D.; Isaacson E.; Cheng A. K. AIDS 2002, 16, 1257. - PubMed
- De Clercq E. Clin. Microbiol. Rev. 2003, 16, 569. - PMC - PubMed
-
- Sofia M. J.; Bao D.; Chang W.; Du J.; Nagarathnam D.; Rachakonda S.; Reddy P. G.; Ross B. S.; Wang P.; Zhang H. R.; Bansal S.; Espiritu C.; Keilman M.; Lam A. M.; Steuer H. M.; Niu C.; Otto M. J.; Furman P. A. J. Med. Chem. 2010, 53, 7202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

