Blocking autocrine VEGF signaling by sunitinib, an anti-cancer drug, promotes embryonic stem cell self-renewal and somatic cell reprogramming
- PMID: 25145356
- PMCID: PMC4152737
- DOI: 10.1038/cr.2014.112
Blocking autocrine VEGF signaling by sunitinib, an anti-cancer drug, promotes embryonic stem cell self-renewal and somatic cell reprogramming
Abstract
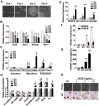
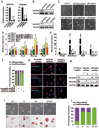
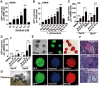
Maintaining the self-renewal of embryonic stem cells (ESCs) could be achieved by activating the extrinsic signaling, i.e., the use of leukemia inhibitory factor (LIF), or blocking the intrinsic differentiation pathways, i.e., the use of GSK3 and MEK inhibitors (2i). Here we found that even in medium supplemented with LIF, mESCs still tend to differentiate toward meso-endoderm lineages after long-term culture and the culture spontaneously secretes vascular endothelial growth factors (VEGFs). Blocking VEGF signaling with sunitinib, an anti-cancer drug and a receptor tyrosine kinase (RTK) inhibitor mainly targeting VEGF receptors (VEGFRs), is capable of maintaining the mESCs in the undifferentiated state without the need for feeder cells or LIF. Sunitinib facilitates the derivation of mESCs from blastocysts, and the mESCs maintained in sunitinib-containing medium remain pluripotent and are able to contribute to chimeric mice. Sunitinib also promotes iPSC generation from MEFs with only Oct4. Knocking down VEGFR2 or blocking it with neutralizing antibody mimicks the effect of sunitinib, indicating that blocking VEGF/VEGFR signaling is indeed beneficial to the self-renewal of mESCs. We also found that hypoxia-inducible factor alpha (HIF1α) and endoplasmic reticulum (ER) stress are involved in the production of VEGF in mESCs. Blocking both pathways inhibits the expression of VEGF and prevents spontaneous differentiation of mESCs. Interestingly, LIF may also exert its effect by downregulating HIF1α and ER stress pathways and subsequent VEGF expression. These results indicate the existence of an intrinsic differentiation pathway in mESCs by activating the autocrine VEGF signaling. Blocking VEGF signaling with sunitinib or other small molecules help to maintain the mESCs in the ground state of pluripotency.
Figures




Similar articles
-
Sunitinib but not VEGF blockade inhibits cancer stem cell endothelial differentiation.Oncotarget. 2015 May 10;6(13):11295-309. doi: 10.18632/oncotarget.3123. Oncotarget. 2015. PMID: 25948774 Free PMC article.
-
Leukemia inhibitory factor-induced Stat3 signaling suppresses fibroblast growth factor 1-induced Erk1/2 activation to inhibit the downstream differentiation in mouse embryonic stem cells.Stem Cells Dev. 2013 Apr 15;22(8):1190-7. doi: 10.1089/scd.2012.0229. Epub 2013 Jan 30. Stem Cells Dev. 2013. PMID: 23205673
-
Conversion from mouse embryonic to extra-embryonic endoderm stem cells reveals distinct differentiation capacities of pluripotent stem cell states.Development. 2012 Aug;139(16):2866-77. doi: 10.1242/dev.078519. Epub 2012 Jul 12. Development. 2012. PMID: 22791892 Free PMC article.
-
Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor.Biochem J. 2011 Aug 15;438(1):11-23. doi: 10.1042/BJ20102152. Biochem J. 2011. PMID: 21793804 Free PMC article. Review.
-
The Art of Capturing Pluripotency: Creating the Right Culture.Stem Cell Reports. 2017 Jun 6;8(6):1457-1464. doi: 10.1016/j.stemcr.2017.05.020. Stem Cell Reports. 2017. PMID: 28591647 Free PMC article. Review.
Cited by
-
Stress signaling in mammalian oocytes and embryos: a basis for intervention and improvement of outcomes.Cell Tissue Res. 2016 Jan;363(1):159-167. doi: 10.1007/s00441-015-2124-9. Epub 2015 Mar 7. Cell Tissue Res. 2016. PMID: 25743689 Free PMC article. Review.
-
Mapping regulators of cell fate determination: Approaches and challenges.APL Bioeng. 2020 Jul 1;4(3):031501. doi: 10.1063/5.0004611. eCollection 2020 Sep. APL Bioeng. 2020. PMID: 32637855 Free PMC article. Review.
-
Sunitinib but not VEGF blockade inhibits cancer stem cell endothelial differentiation.Oncotarget. 2015 May 10;6(13):11295-309. doi: 10.18632/oncotarget.3123. Oncotarget. 2015. PMID: 25948774 Free PMC article.
-
Epithelioid peritoneal mesothelioma: a hybrid phenotype within a mesenchymal-epithelial/epithelial-mesenchymal transition framework.Oncotarget. 2016 Nov 15;7(46):75503-75517. doi: 10.18632/oncotarget.12262. Oncotarget. 2016. PMID: 27705913 Free PMC article.
-
Analysis for Carom complex, signaling and function by database mining.Front Biosci (Landmark Ed). 2016 Jan 1;21(4):856-72. doi: 10.2741/4424. Front Biosci (Landmark Ed). 2016. PMID: 26709809 Free PMC article.
References
-
- Ulloa-Montoya F, Verfaillie CM, Hu WS. Culture systems for pluripotent stem cells. J Biosci Bioeng. 2005;100:12–27. - PubMed
-
- Williams RL, Hilton DJ, Pease S, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. - PubMed
-
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. - PubMed
-
- Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

