Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis
- PMID: 25145391
- PMCID: PMC4150933
- DOI: 10.1038/emm.2014.49
Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis
Abstract
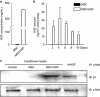
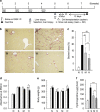
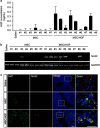
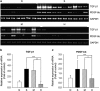
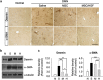
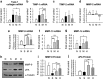
Bone marrow-derived mesenchymal stromal cells (MSCs) have been reported to be beneficial for the treatment of liver fibrosis. Here, we investigated the use of genetically engineered MSCs that overexpress hepatocyte growth factor (HGF) as a means to improve their therapeutic effect in liver fibrosis. Liver fibrosis was induced by intraperitoneal injection of dimethylnitrosamine. HGF-secreting MSCs (MSCs/HGF) were prepared by transducing MSCs with an adenovirus carrying HGF-encoding cDNA. MSCs or MSCs/HGF were injected directly into the spleen of fibrotic rats. Tissue fibrosis was assessed by histological analysis 12 days after stem cell injection. Although treatment with MSCs reduced fibrosis, treatment with MSCs/HGF produced a more significant reduction and was associated with elevated HGF levels in the portal vein. Collagen levels in the liver extract were decreased after MSC/HGF therapy, suggesting recovery from fibrosis. Furthermore, liver function was improved in animals receiving MSCs/HGF, indicating that MSC/HGF therapy resulted not only in reduction of liver fibrosis but also in improvement of hepatocyte function. Assessment of cell and biochemical parameters revealed that mRNA levels of the fibrogenic cytokines PDGF-bb and TGF-β1 were significantly decreased after MSC/HGF therapy. Subsequent to the decrease in collagen, expression of matrix metalloprotease-9 (MMP-9), MMP-13, MMP-14 and urokinase-type plasminogen activator was augmented following MSC/HGF, whereas tissue inhibitor of metalloprotease-1 (TIMP-1) expression was reduced. In conclusion, therapy with MSCs/HGF resulted in an improved therapeutic effect compared with MSCs alone, probably because of the anti-fibrotic activity of HGF. Thus, MSC/HGF represents a promising approach toward a cell therapy for liver fibrosis.
Figures

References
-
- Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. - PubMed
-
- Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83–88. - PubMed
-
- Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, et al. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742–748. - PubMed
-
- Ong SY, Dai H, Leong KW. Hepatic differentiation potential of commercially available human mesenchymal stem cells. Tissue Eng. 2006;12:3477–3485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
