Early changes of muscle membrane properties in porcine faecal peritonitis
- PMID: 25145497
- PMCID: PMC4159512
- DOI: 10.1186/s13054-014-0484-2
Early changes of muscle membrane properties in porcine faecal peritonitis
Abstract
Introduction: Sepsis-induced myopathy and critical illness myopathy (CIM) are possible causes of muscle weakness in intensive care patients. They have been attributed to muscle membrane dysfunction. The aim of this study was to investigate membrane properties in the early stage of experimental sepsis by evaluating muscle excitability.
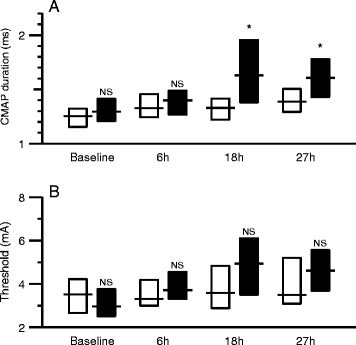
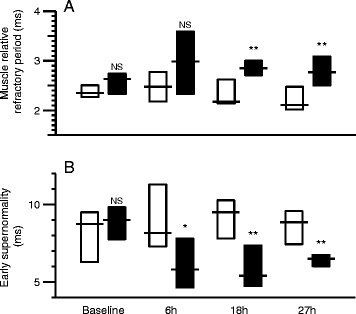
Methods: In total, 20 anesthetized and mechanically ventilated pigs were randomized to either faecal peritonitis (n = 10) or to non-septic controls (n = 10). Resuscitation with fluids and vasoactive drugs was started 3 hours after peritonitis induction. Muscle membrane properties were investigated by measuring muscle velocity recovery cycles before induction of peritonitis as well as 6, 18 and 27 hours thereafter. Muscle relative refractory period (MRRP) and early supernormality (ESN) were assessed.
Results: Peritonitis lasting 27 hours was associated with an increase of MRRP by 28% from 2.38 ± 0.18 ms (mean ± SD) to 3.47 ± 1.79 ms (P <0.01) and a decrease of ESN by 31% from 9.64 ± 2.82% to 6.50 ± 2.64% (P <0.01). ESN reduction was already apparent 6 hours after induction of peritonitis. Values in controls did not show any significant alterations.
Conclusions: Muscle membrane abnormalities consistent with membrane depolarization and/or sodium channel inactivation occurred within 6 hours of peritonitis induction. This indicates that changes that have been described in established sepsis-induced myopathy and/or CIM start early in the course of sepsis. Muscle excitability testing facilitates evaluation of the time course of these changes.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

