RIP kinases: key decision makers in cell death and innate immunity
- PMID: 25146926
- PMCID: PMC4291486
- DOI: 10.1038/cdd.2014.126
RIP kinases: key decision makers in cell death and innate immunity
Abstract
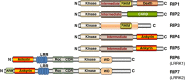
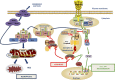
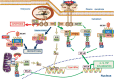
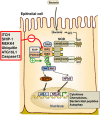
Innate immunity represents the first line of defence against invading pathogens. It consists of an initial inflammatory response that recruits white blood cells to the site of infection in an effort to destroy and eliminate the pathogen. Some pathogens replicate within host cells, and cell death by apoptosis is an important effector mechanism to remove the replication niche for such microbes. However, some microbes have evolved evasive strategies to block apoptosis, and in these cases host cells may employ further countermeasures, including an inflammatory form of cell death know as necroptosis. This review aims to highlight the importance of the RIP kinase family in controlling these various defence strategies. RIP1 is initially discussed as a key component of death receptor signalling and in the context of dictating whether a cell triggers a pathway of pro-inflammatory gene expression or cell death by apoptosis. The molecular and functional interplay of RIP1 and RIP3 is described, especially with respect to mediating necroptosis and as key mediators of inflammation. The function of RIP2, with particular emphasis on its role in NOD signalling, is also explored. Special attention is given to emphasizing the physiological and pathophysiological contexts for these various functions of RIP kinases.
Figures
Comment in
-
Targeting disease by immunomodulation.Cell Death Differ. 2015 Feb;22(2):185-6. doi: 10.1038/cdd.2014.166. Cell Death Differ. 2015. PMID: 25578147 Free PMC article. No abstract available.
References
-
- O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. - PubMed
-
- Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–398. - PubMed
-
- Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

