The interaction of cytoplasmic poly(A)-binding protein with eukaryotic initiation factor 4G suppresses nonsense-mediated mRNA decay
- PMID: 25147240
- PMCID: PMC4174440
- DOI: 10.1261/rna.044933.114
The interaction of cytoplasmic poly(A)-binding protein with eukaryotic initiation factor 4G suppresses nonsense-mediated mRNA decay
Abstract
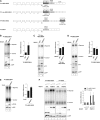
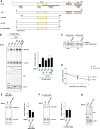
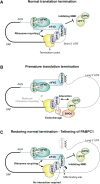
Nonsense-mediated mRNA decay (NMD) eliminates different classes of mRNA substrates including transcripts with long 3' UTRs. Current models of NMD suggest that the long physical distance between the poly(A) tail and the termination codon reduces the interaction between cytoplasmic poly(A)-binding protein (PABPC1) and the eukaryotic release factor 3a (eRF3a) during translation termination. In the absence of PABPC1 binding, eRF3a recruits the NMD factor UPF1 to the terminating ribosome, triggering mRNA degradation. Here, we have used the MS2 tethering system to investigate the suppression of NMD by PABPC1. We show that tethering of PABPC1 between the termination codon and a long 3' UTR specifically inhibits NMD-mediated mRNA degradation. Contrary to the current model, tethered PABPC1 mutants unable to interact with eRF3a still efficiently suppress NMD. We find that the interaction of PABPC1 with eukaryotic initiation factor 4G (eIF4G), which mediates the circularization of mRNAs, is essential for NMD inhibition by tethered PABPC1. Furthermore, recruiting either eRF3a or eIF4G in proximity to an upstream termination codon antagonizes NMD. While tethering of an eRF3a mutant unable to interact with PABPC1 fails to suppress NMD, tethered eIF4G inhibits NMD in a PABPC1-independent manner, indicating a sequential arrangement of NMD antagonizing factors. In conclusion, our results establish a previously unrecognized link between translation termination, mRNA circularization, and NMD suppression, thereby suggesting a revised model for the activation of NMD at termination codons upstream of long 3' UTR.
Keywords: NMD; PABPC1; eIF4G; ribosome recycling; translation termination.
© 2014 Fatscher et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures




References
-
- Amrani N, Ganesan R, Kervestin S, Mangus DA, Ghosh S, Jacobson A 2004. A faux 3′-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature 432: 112–118 - PubMed
-
- Bhuvanagiri M, Schlitter AM, Hentze MW, Kulozik AE 2010. NMD: RNA biology meets human genetic medicine. Biochem J 430: 365–377 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
