Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function
- PMID: 25147279
- PMCID: PMC4869326
- DOI: 10.1126/science.1252945
Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function
Abstract
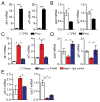
Aging-associated cognitive decline is affected by factors produced inside and outside the brain. By using multiorgan genome-wide analysis of aged mice, we found that the choroid plexus, an interface between the brain and the circulation, shows a type I interferon (IFN-I)-dependent gene expression profile that was also found in aged human brains. In aged mice, this response was induced by brain-derived signals, present in the cerebrospinal fluid. Blocking IFN-I signaling within the aged brain partially restored cognitive function and hippocampal neurogenesis and reestablished IFN-II-dependent choroid plexus activity, which is lost in aging. Our data identify a chronic aging-induced IFN-I signature, often associated with antiviral response, at the brain's choroid plexus and demonstrate its negative influence on brain function, thereby suggesting a target for ameliorating cognitive decline in aging.
Copyright © 2014, American Association for the Advancement of Science.
Figures
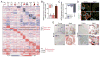


Comment in
-
Physiology. Good barriers make good neighbors.Science. 2014 Oct 3;346(6205):36-7. doi: 10.1126/science.1260705. Epub 2014 Oct 2. Science. 2014. PMID: 25278599 No abstract available.
References
-
- Yeoman M, Scutt G, Faragher R. Nat Rev Neurosci. 2012;13:435–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

