Ion mobility spectrometry: A personal view of its development at UCSB
- PMID: 25147478
- PMCID: PMC4135396
- DOI: 10.1016/j.ijms.2014.06.016
Ion mobility spectrometry: A personal view of its development at UCSB
Abstract
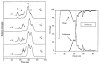
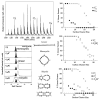
Ion mobility is not a newly discovered phenomenon. It has roots going back to Langevin at the beginning of the 20th century. Our group initially got involved by accident around 1990 and this paper is a brief account of what has transpired here at UCSB the past 25 years in response to this happy accident. We started small, literally, with transition metal atomic ions and transitioned to carbon clusters, synthetic polymers, most types of biological molecules and eventually peptide and protein oligomeric assembly. Along the way we designed and built several generations of instruments, a process that is still ongoing. And perhaps most importantly we have incorporated theory with experiment from the beginning; a necessary wedding that allows an atomistic face to be put on the otherwise interesting but not fully informative cross section measurements.
Keywords: Bio-macromolecules; Instrumentation; Ion mobility; Mass spectrometry; Modeling; Structure.
Figures


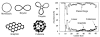





Similar articles
-
Cryogenic Ion Mobility-Mass Spectrometry: Tracking Ion Structure from Solution to the Gas Phase.Acc Chem Res. 2016 Jul 19;49(7):1421-8. doi: 10.1021/acs.accounts.6b00177. Epub 2016 Jun 23. Acc Chem Res. 2016. PMID: 27334393
-
Ion mobility mass spectrometry of proteins and protein assemblies.Chem Soc Rev. 2010 May;39(5):1633-55. doi: 10.1039/b914002f. Epub 2009 Nov 25. Chem Soc Rev. 2010. PMID: 20419213 Review.
-
Effective collisional cross-section of small ions in the gas phase: Application to ion mobility spectrometry.Rapid Commun Mass Spectrom. 2021 Jun 30;35(12):e9090. doi: 10.1002/rcm.9090. Rapid Commun Mass Spectrom. 2021. PMID: 33760281
-
Polymers for Traveling Wave Ion Mobility Spectrometry Calibration.J Am Soc Mass Spectrom. 2017 Nov;28(11):2483-2491. doi: 10.1007/s13361-017-1762-4. Epub 2017 Jul 31. J Am Soc Mass Spectrom. 2017. PMID: 28762031
-
Continuous-flow differential mobility analysis of nanoparticles and biomolecules.Annu Rev Chem Biomol Eng. 2014;5:255-79. doi: 10.1146/annurev-chembioeng-061312-103316. Epub 2014 Mar 20. Annu Rev Chem Biomol Eng. 2014. PMID: 24655136 Review.
Cited by
-
The Solution Assembly of Biological Molecules Using Ion Mobility Methods: From Amino Acids to Amyloid β-Protein.Annu Rev Anal Chem (Palo Alto Calif). 2017 Jun 12;10(1):365-386. doi: 10.1146/annurev-anchem-071114-040304. Epub 2017 Mar 24. Annu Rev Anal Chem (Palo Alto Calif). 2017. PMID: 28375705 Free PMC article. Review.
-
Linking molecular models with ion mobility experiments. Illustration with a rigid nucleic acid structure.J Mass Spectrom. 2015 May;50(5):711-26. doi: 10.1002/jms.3590. J Mass Spectrom. 2015. PMID: 26259654 Free PMC article.
-
Separation and Identification of Glycan Anomers Using Ultrahigh-Resolution Ion-Mobility Spectrometry and Cryogenic Ion Spectroscopy.J Am Soc Mass Spectrom. 2019 Nov;30(11):2204-2211. doi: 10.1007/s13361-019-02333-0. Epub 2019 Sep 13. J Am Soc Mass Spectrom. 2019. PMID: 31520337
-
Implementation of charged microdroplet-based derivatization of bile acids on a cyclic ion mobility spectrometry-mass spectrometry platform.Anal Methods. 2023 Nov 2;15(42):5577-5581. doi: 10.1039/d3ay01447a. Anal Methods. 2023. PMID: 37853730 Free PMC article.
-
DNA Binding and Phosphorylation Regulate the Core Structure of the NF-κB p50 Transcription Factor.J Am Soc Mass Spectrom. 2019 Jan;30(1):128-138. doi: 10.1007/s13361-018-1984-0. Epub 2018 Jun 5. J Am Soc Mass Spectrom. 2019. PMID: 29873020 Free PMC article.
References
-
- Franklin B. A letter of Benjamin Franklin, Esq; to Mr. Peter Collinson, F. R. S. concerning an electrical kite. Phil. Trans. 1751;47:565–567.
-
- Coulomb CA. Mém. de l'Acad. Vol. 616. Paris: 1795.
-
- Thomson JJ, Rutherford E. On the passage of electricity through gases exposed to Röntgen rays. Philos. Mag. 1896;42:392–407.
-
- Rutherford E. The velocity and rate of recombination of the ions of gases exposed to Röntgen radiation. Philos. Mag. 1897;44:422–440.
-
- Langevin P. Sur la mobilité des ions les gaz. Comptes Rendus. 1902;134:646–649.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
