1,3,4-oxadiazole derivatives: synthesis, characterization, antimicrobial potential, and computational studies
- PMID: 25147788
- PMCID: PMC4131560
- DOI: 10.1155/2014/172791
1,3,4-oxadiazole derivatives: synthesis, characterization, antimicrobial potential, and computational studies
Abstract
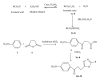
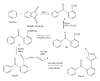
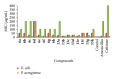
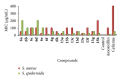
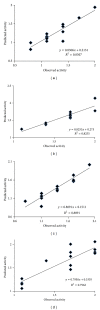
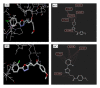
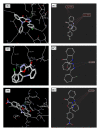
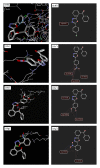
We report the synthesis and biological assessment of 1,3,4-oxadiazole substituted 24 derivatives as novel, potential antibacterial agents. The structures of the newly synthesized derivatives were established by the combined practice of UV, IR, (1)H NMR, (13)C NMR, and mass spectrometry. Further these synthesized derivatives were subjected to antibacterial activity against all the selected microbial strains in comparison with amoxicillin and cefixime. The antibacterial activity of synthesized derivatives was correlated with their physicochemical and structural properties by QSAR analysis using computer assisted multiple regression analysis and four sound predictive models were generated with good R(2), R(adj)(2), and Fischer statistic. The derivatives with potent antibacterial activity were subjected to molecular docking studies to investigate the interactions between the active derivatives and amino acid residues existing in the active site of peptide deformylase to assess their antibacterial potential as peptide deformylase inhibitor.
Figures

References
-
- Sengupta P, Mal M, Mandal S, Singh J, Maity TK. Evaluation of antibacterial and antifungal activity of some 1, 3, 4 oxadiazoles. Iranian Journal of Pharmacology and Therapeutics. 2008;7(2):165–167.
-
- Bhardwaj N, Saraf SK, Sharma P, Kumar P. Syntheses, evaluation and characterization of some 1, 3, 4-oxadiazoles as antimicrobial agents. E-Journal of Chemistry. 2009;6(4):1133–1138.
-
- Kadi AA, El-Brollosy NR, Al-Deeb OA, Habib EE, Ibrahim TM, El-Emam AA. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. European Journal of Medicinal Chemistry. 2007;42(2):235–242. - PubMed
-
- Mickevičius V, Vaickelioniene R, Sapijanskaite B. Synthesis of substituted 1,3,4-oxadiazole derivatives. Chemistry of Heterocyclic Compounds. 2009;45(2):215–218.
-
- Bentiss F, Lagrenee M. A new synthesis of symmetrical 2,5-disubstituted 1,3,4-oxadiazoles. Journal of Heterocyclic Chemistry. 1999;36(4):1029–1032.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

