The effect of rilonacept versus placebo on health-related quality of life in patients with poorly controlled familial Mediterranean fever
- PMID: 25147819
- PMCID: PMC4131422
- DOI: 10.1155/2014/854842
The effect of rilonacept versus placebo on health-related quality of life in patients with poorly controlled familial Mediterranean fever
Abstract
Objective: To examine the effect of rilonacept on the health-related quality of life (HRQoL) in patients with poorly controlled familial Mediterranean fever (FMF).
Methods: As part of a randomized, double-blinded trial comparing rilonacept and placebo for the treatment of FMF, patients/parents completed the modified Child Health Questionnaire (CHQ) at baseline, and at the start and end of each of 4 treatment courses, 2 each with rilonacept and placebo.
Results: Fourteen subjects were randomized; mean age was 24.4 ± 11.8 years. At baseline the physical HRQoL score was significantly less (24.2 ± 49.5) but the psychosocial score was similar to the population norm (49.5 ± 10.0). There were significant improvements in most HRQoL concepts after rilonacept but not placebo. Significant differences between rilonacept and placebo were found in the physical (33.7 ± 16.4 versus 23.7 ± 14.5, P = 0.021) but not psychosocial scores (51.4 ± 10.3 versus 49.8 ± 12.4, P = 0.42). The physical HRQoL was significantly impacted by the treatment effect and patient global assessment.
Conclusion: Treatment with rilonacept had a beneficial effect on the physical HRQoL in patients with poorly controlled FMF and was also significantly related to the patient global assessment. This trial is registered with ClinicalTrials.gov Identifier NCT00582907.
Figures
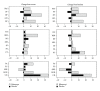
References
-
- Sohar E, Gafni J, Pras M, Heller H. Familial Mediterranean fever. A survey of 470 cases and review of the literature. The American Journal of Medicine. 1967;43(2):227–253. - PubMed
-
- The International FMF Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997;90:797–807. - PubMed
-
- The French FMF Consortium. A candidate gene for familial Mediterranean fever. Nature Genetics. 1997;17:25–31. - PubMed
-
- Ben-Chetrit E, Touitou I. Familial Mediterranean fever in the world. Arthritis Care and Research. 2009;61(10):1447–1453. - PubMed
-
- Goldfinger SE. Colchicine for familial Mediterranean fever. The New England Journal of Medicine. 1972;287(25):p. 1302. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

