L-type Ca2+ channel responses to bay k 8644 in stem cell-derived cardiomyocytes are unusually dependent on holding potential and charge carrier
- PMID: 25147907
- PMCID: PMC4142808
- DOI: 10.1089/adt.2014.596
L-type Ca2+ channel responses to bay k 8644 in stem cell-derived cardiomyocytes are unusually dependent on holding potential and charge carrier
Abstract
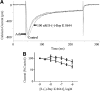
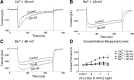
Human stem cell-derived cardiomyocytes provide a cellular model for the study of electrophysiology in the human heart and are finding a niche in the field of safety pharmacology for predicting proarrhythmia. The cardiac L-type Ca2+ channel is an important target for some of these safety studies. However, the pharmacology of this channel in these cells is altered compared to native cardiac tissue, specifically in its sensitivity to the Ca2+ channel activator S-(-)-Bay K 8644. Using patch clamp electrophysiology, we examined the effects of S-(-)-Bay K 8644 in three separate stem cell-derived cardiomyocyte cell lines under various conditions in an effort to detect more typical responses to the drug. S-(-)-Bay K 8644 failed to produce characteristically large increases in current when cells were held at -40 mV and Ca2+ was used as the charge carrier, although high-affinity binding and the effects of the antagonist isomer, R-(+)-Bay K 8644, were intact. Dephosphorylation of the channel with acetylcholine failed to restore the sensitivity of the channel to the drug. Only when the holding potential was shifted to a more hyperpolarized (-60 mV) level, and external Ca2+ was replaced by Ba2+, could large increases in current amplitude be observed. Even under these conditions, increases in current amplitude varied dramatically between different cell lines and channel kinetics following drug addition were generally atypical. The results indicate that the pharmacology of S-(-)-Bay K 8644 in stem cell-derived cardiomyocytes varies by cell type, is unusually dependent on holding potential and charge carrier, and is different from that observed in primary human heart cells.
Figures

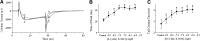



Similar articles
-
Effects of the enantiomers of BayK 8644 on the charge movement of L-type Ca channels in guinea-pig ventricular myocytes.J Membr Biol. 2003 Jun 1;193(3):215-27. doi: 10.1007/s00232-003-2020-1. J Membr Biol. 2003. PMID: 12962282
-
Ca²⁺ channel activators reveal differential L-type Ca²⁺ channel pharmacology between native and stem cell-derived cardiomyocytes.J Pharmacol Exp Ther. 2012 May;341(2):510-7. doi: 10.1124/jpet.112.192609. Epub 2012 Feb 21. J Pharmacol Exp Ther. 2012. PMID: 22353878
-
Competitive and cooperative effects of Bay K8644 on the L-type calcium channel current inhibition by calcium channel antagonists.J Pharmacol Exp Ther. 2007 Aug;322(2):638-45. doi: 10.1124/jpet.107.122176. Epub 2007 May 2. J Pharmacol Exp Ther. 2007. PMID: 17475903
-
[Single channel analysis of Ca2+ channel-agonistic action of dihydropyridine derivatives: voltage-dependent effects of YC-170 and BAY K 8644].Hokkaido Igaku Zasshi. 1993 Jul;68(4):557-69. Hokkaido Igaku Zasshi. 1993. PMID: 7687975 Japanese.
-
L-type calcium channels in vascular smooth muscle cells from spontaneously hypertensive rats: effects of calcium agonist and antagonist.Hypertens Res. 1998 Mar;21(1):33-7. doi: 10.1291/hypres.21.33. Hypertens Res. 1998. PMID: 9582106
Cited by
-
Ca(2+)-Currents in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Effects of Two Different Culture Conditions.Front Pharmacol. 2016 Sep 12;7:300. doi: 10.3389/fphar.2016.00300. eCollection 2016. Front Pharmacol. 2016. PMID: 27672365 Free PMC article.
-
Excitation-contraction coupling of human induced pluripotent stem cell-derived cardiomyocytes.Front Cell Dev Biol. 2015 Sep 29;3:59. doi: 10.3389/fcell.2015.00059. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26484342 Free PMC article. Review.
-
Human Engineered Heart Tissue: Analysis of Contractile Force.Stem Cell Reports. 2016 Jul 12;7(1):29-42. doi: 10.1016/j.stemcr.2016.04.011. Epub 2016 May 19. Stem Cell Reports. 2016. PMID: 27211213 Free PMC article.
References
-
- Triggle DJ: The pharmacology of ion channels: with particular reference to voltage-gated Ca2+ channels. Eur J Pharmacol 1999;375:311–325 - PubMed
-
- Zhang S, Zhou Z, Gong Q, Makielski JC, January CT: Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ Res 1999;84:989–998 - PubMed
-
- Kang J, Chen X-L, Wang H, et al. : Cardiac ion channel effects of tolterodine. J Pharmacol Exp Ther 2004;308:935–940 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
