Protonation states of the tryptophan synthase internal aldimine active site from solid-state NMR spectroscopy: direct observation of the protonated Schiff base linkage to pyridoxal-5'-phosphate
- PMID: 25148001
- PMCID: PMC4183654
- DOI: 10.1021/ja506267d
Protonation states of the tryptophan synthase internal aldimine active site from solid-state NMR spectroscopy: direct observation of the protonated Schiff base linkage to pyridoxal-5'-phosphate
Abstract
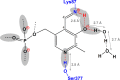
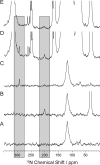
The acid-base chemistry that drives catalysis in pyridoxal-5'-phosphate (PLP)-dependent enzymes has been the subject of intense interest and investigation since the initial identification of PLP's role as a coenzyme in this extensive class of enzymes. It was first proposed over 50 years ago that the initial step in the catalytic cycle is facilitated by a protonated Schiff base form of the holoenzyme in which the linking lysine ε-imine nitrogen, which covalently binds the coenzyme, is protonated. Here we provide the first (15)N NMR chemical shift measurements of such a Schiff base linkage in the resting holoenzyme form, the internal aldimine state of tryptophan synthase. Double-resonance experiments confirm the assignment of the Schiff base nitrogen, and additional (13)C, (15)N, and (31)P chemical shift measurements of sites on the PLP coenzyme allow a detailed model of coenzyme protonation states to be established.
Figures
Similar articles
-
Catalytic roles of βLys87 in tryptophan synthase: (15)N solid state NMR studies.Biochim Biophys Acta. 2015 Sep;1854(9):1194-9. doi: 10.1016/j.bbapap.2015.02.003. Epub 2015 Feb 14. Biochim Biophys Acta. 2015. PMID: 25688830 Free PMC article.
-
Allosteric regulation of tryptophan synthase: effects of pH, temperature, and alpha-subunit ligands on the equilibrium distribution of pyridoxal 5'-phosphate-L-serine intermediates.Biochemistry. 1996 Feb 13;35(6):1872-80. doi: 10.1021/bi951889c. Biochemistry. 1996. PMID: 8639669
-
NMR studies of the stability, protonation States, and tautomerism of (13)C- AND (15)N-labeled aldimines of the coenzyme pyridoxal 5'-phosphate in water.Biochemistry. 2010 Dec 28;49(51):10818-30. doi: 10.1021/bi101061m. Epub 2010 Dec 6. Biochemistry. 2010. PMID: 21067170
-
Stereospecificity of alpha-proton exchange reactions catalysed by pyridoxal-5'-phosphate-dependent enzymes.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):138-42. doi: 10.1016/s1570-9639(03)00080-3. Biochim Biophys Acta. 2003. PMID: 12686123 Review.
-
Critical hydrogen bonds and protonation states of pyridoxal 5'-phosphate revealed by NMR.Biochim Biophys Acta. 2011 Nov;1814(11):1426-37. doi: 10.1016/j.bbapap.2011.06.004. Epub 2011 Jun 16. Biochim Biophys Acta. 2011. PMID: 21703367 Review.
Cited by
-
NMR Crystallography of a Carbanionic Intermediate in Tryptophan Synthase: Chemical Structure, Tautomerization, and Reaction Specificity.J Am Chem Soc. 2016 Nov 23;138(46):15214-15226. doi: 10.1021/jacs.6b08937. Epub 2016 Nov 11. J Am Chem Soc. 2016. PMID: 27779384 Free PMC article.
-
Synthesis of [13C₃]-B6 Vitamers Labelled at Three Consecutive Positions Starting from [13C₃]-Propionic Acid.Molecules. 2018 Aug 23;23(9):2117. doi: 10.3390/molecules23092117. Molecules. 2018. PMID: 30142892 Free PMC article.
-
Engineered Biocatalytic Synthesis of β-N-Substituted-α-Amino Acids.Angew Chem Int Ed Engl. 2023 Oct 23;62(43):e202311189. doi: 10.1002/anie.202311189. Epub 2023 Sep 14. Angew Chem Int Ed Engl. 2023. PMID: 37625129 Free PMC article.
-
Solution-State (17)O Quadrupole Central-Transition NMR Spectroscopy in the Active Site of Tryptophan Synthase.Angew Chem Int Ed Engl. 2016 Jan 22;55(4):1350-4. doi: 10.1002/anie.201508898. Epub 2015 Dec 10. Angew Chem Int Ed Engl. 2016. PMID: 26661504 Free PMC article.
-
Investigation of Structural Dynamics of Enzymes and Protonation States of Substrates Using Computational Tools.Catalysts. 2016 Jun;6(6):82. doi: 10.3390/catal6060082. Epub 2016 May 31. Catalysts. 2016. PMID: 27885336 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

