The effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trial
- PMID: 25148049
- PMCID: PMC4141795
- DOI: 10.1371/journal.pone.0105609
The effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trial
Abstract
Background: Chronic Obstructive Pulmonary Disease (COPD) is a progressive airway disease characterised by neutrophilic airway inflammation or bronchitis. Neutrophilic bronchitis is associated with both bacterial colonisation and lung function decline and is common in exacerbations of COPD. Despite current available therapies to control inflammation, neutrophilic bronchitis remains common. This study tested the hypothesis that azithromycin treatment, as an add-on to standard medication, would significantly reduce airway neutrophil and neutrophils chemokine (CXCL8) levels, as well as bacterial load. We conducted a randomised, double-blind, placebo-controlled study in COPD participants with stable neutrophilic bronchitis.
Methods: Eligible participants (n = 30) were randomised to azithromycin 250 mg daily or placebo for 12 weeks in addition to their standard respiratory medications. Sputum was induced at screening, randomisation and monthly for a 12 week treatment period and processed for differential cell counts, CXCL8 and neutrophil elastase assessment. Quantitative bacteriology was assessed in sputum samples at randomisation and the end of treatment visit. Severe exacerbations where symptoms increased requiring unscheduled treatment were recorded during the 12 week treatment period and for 14 weeks following treatment. A sub-group of participants underwent chest computed tomography scans (n = 15).
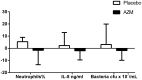
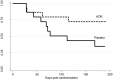
Results: Nine participants with neutrophilic bronchitis had a potentially pathogenic bacteria isolated and the median total bacterial load of all participants was 5.22×107 cfu/mL. Azithromycin treatment resulted in a non-significant reduction in sputum neutrophil proportion, CXCL8 levels and bacterial load. The mean severe exacerbation rate was 0.33 per person per 26 weeks in the azithromycin group compared to 0.93 exacerbations per person in the placebo group (incidence rate ratio (95%CI): 0.37 (0.11,1.21), p = 0.062). For participants who underwent chest CT scans, no alterations were observed.
Conclusions: In stable COPD with neutrophilic bronchitis, add-on azithromycin therapy showed a trend to reduced severe exacerbations sputum neutrophils, CXCL8 levels and bacterial load. Future studies with a larger sample size are warranted.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12609000259246.
Conflict of interest statement
Figures

References
-
- Simpson JL, Gibson PG, Yang IA, Upham J, James A, et al. (2013) Impaired macrophage phagocytosis in non-eosinophilic asthma. Clin Exp Allergy 43: 29–35. - PubMed
-
- Simpson JL, Phipps S, Gibson PG (2009) Inflammatory mechanisms and treatment of obstructive airway diseases with neutrophilic bronchitis. Pharmacology & Therapeutics 124: 86–95. - PubMed
-
- Wilkinson TMA, Patel IS, Wilks M, Donaldson GC, Wedzicha JA (2003) Airway Bacterial Load and FEV1 Decline in Patients with Chronic Obstructive Pulmonary Disease 10.1164/rccm.200210-1179OC. Am J Respir Crit Care Med 167: 1090–1095. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

