ACK1 tyrosine kinase interacts with histone demethylase KDM3A to regulate the mammary tumor oncogene HOXA1
- PMID: 25148682
- PMCID: PMC4192474
- DOI: 10.1074/jbc.M114.584425
ACK1 tyrosine kinase interacts with histone demethylase KDM3A to regulate the mammary tumor oncogene HOXA1
Abstract
Hormone therapy with the selective estrogen-receptor modulator tamoxifen provides a temporary relief for patients with estrogen receptor α (ER)-positive breast cancers. However, a subset of patients exhibiting overexpression of the HER2 receptor tyrosine kinase displays intrinsic resistance to tamoxifen therapy. Therefore, elucidating the mechanisms promoting the estrogen (E2)-independent ER-regulated gene transcription in tamoxifen-resistant breast tumors is essential to identify new therapeutic avenues to overcome drug resistance and ameliorate poor prognosis. The non-receptor tyrosine kinase, ACK1 (also known as TNK2), has emerged as a major integrator of signaling from various receptor tyrosine kinases including HER2. We have uncovered that heregulin-mediated ACK1 activation promoted ER activity in the presence of tamoxifen, which was significantly down-regulated upon ACK1 knockdown or inhibition of ACK1 by small molecule inhibitors, AIM-100 or Dasatinib. We report that ACK1 phosphorylates the ER co-activator, KDM3A, a H3K9 demethylase, at an evolutionary conserved tyrosine 1114 site in a heregulin-dependent manner, even in the presence of tamoxifen. Consistent with this finding, ACK1 activation resulted in a significant decrease in the deposition of dimethyl H3K9 epigenetic marks. Conversely, inhibition of ACK1 by AIM-100 or Dasatinib restored dimethyl H3K9 methylation marks and caused transcriptional suppression of the ER-regulated gene HOXA1. Thus, by its ability to regulate the epigenetic activity of an ER co-activator KDM3A, ACK1 modulates HOXA1 expression in the absence of E2, conferring tamoxifen resistance. These data reveal a novel therapeutic option, suppression of ACK1 signaling by AIM-100 or Dasatinib, to mitigate HOXA1 up-regulation in breast cancer patients displaying tamoxifen resistance.
Keywords: Breast Cancer; Cancer Therapy; Oncogene; Signal Transduction; Transcription Regulation; Tyrosine-Protein Kinase (Protein-tyrosine Kinase).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
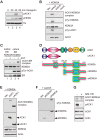
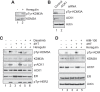



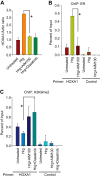
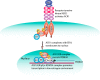
References
-
- Parkin D. M., Bray F., Ferlay J., Pisani P. (2005) Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 - PubMed
-
- Parkin D. M., Bray F., Ferlay J., Pisani P. (2001) Estimating the world cancer burden: Globocan 2000. Int. J. Cancer 94, 153–156 - PubMed
-
- Green K. A., Carroll J. S. (2007) Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat. Rev. Cancer 7, 713–722 - PubMed
-
- Fisher B., Costantino J. P., Wickerham D. L., Redmond C. K., Kavanah M., Cronin W. M., Vogel V., Robidoux A., Dimitrov N., Atkins J., Daly M., Wieand S., Tan-Chiu E., Ford L., Wolmark N. (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 90, 1371–1388 - PubMed
-
- Fisher B., Costantino J. P., Wickerham D. L., Cecchini R. S., Cronin W. M., Robidoux A., Bevers T. B., Kavanah M. T., Atkins J. N., Margolese R. G., Runowicz C. D., James J. M., Ford L. G., Wolmark N. (2005) Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl. Cancer Inst. 97, 1652–1662 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

