Effect of substrate mechanics on cardiomyocyte maturation and growth
- PMID: 25148904
- PMCID: PMC4321772
- DOI: 10.1089/ten.TEB.2014.0383
Effect of substrate mechanics on cardiomyocyte maturation and growth
Erratum in
-
Correction Re: Tissue Engineering, Part B, 2015;21(1):157-165.Tissue Eng Part B Rev. 2016 Jun;22(3):263. doi: 10.1089/ten.teb.2014.0383.correx. Tissue Eng Part B Rev. 2016. PMID: 27227520 Free PMC article. No abstract available.
Abstract
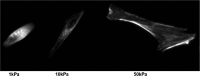
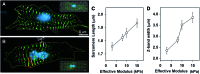
Cardiac tissue engineering constructs are a promising therapeutic treatment for myocardial infarction, which is one of the leading causes of death. In order to further advance the development and regeneration of engineered cardiac tissues using biomaterial platforms, it is important to have a complete overview of the effects that substrates have on cardiomyocyte (CM) morphology and function. This article summarizes recent studies that investigate the effect of mechanical cues on the CM differentiation, maturation, and growth. In these studies, CMs derived from embryos, neonates, and mesenchymal stem cells were seeded on different substrates of various elastic modulus. Measuring the contractile function by force production, work output, and calcium handling, it was seen that cell behavior on substrates was optimized when the substrate stiffness mimicked that of the native tissue. The contractile function reflected changes in the sarcomeric protein confirmation and organization that promoted the contractile ability. The analysis of the literature also revealed that, in addition to matrix stiffness, mechanical stimulation, such as stretching the substrate during cell seeding, also played an important role during cell maturation and tissue development.
Figures





References
-
- Patel J.K., and Kobashigawa J.A.Cardiology patient page. Heart transplantation. Circulation 124,E132, 2011 - PubMed
-
- Jawad H., Lyon A., Harding S.E., Ali N., and Boccaccini A.Myocardial tissue engineering. Br Med Bull 87,31, 2008 - PubMed
-
- Soonpaa M.H., and Field L.J.Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res 83,15, 1998 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

