Expression of 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNPase) and its roles in activated microglia in vivo and in vitro
- PMID: 25148928
- PMCID: PMC4244045
- DOI: 10.1186/s12974-014-0148-9
Expression of 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNPase) and its roles in activated microglia in vivo and in vitro
Abstract
Background: We reported previously that amoeboid microglial cells in the postnatal rat brain expressed 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNPase) both in vivo and in vitro; however, the functional role of CNPase in microglia has remained uncertain. This study extended the investigation to determine CNPase expression in activated microglia derived from cell culture and animal models of brain injury with the objective to clarify its putative functions.
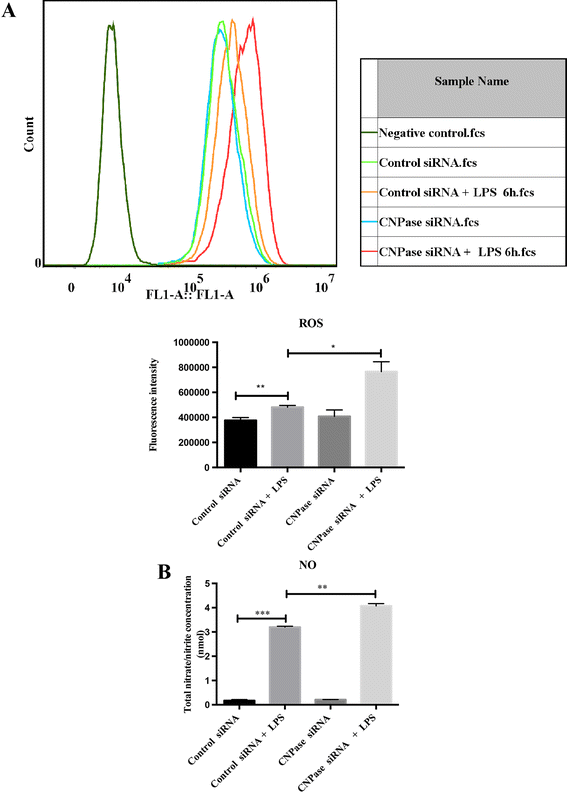
Methods: Three-day-old Wistar rats were given an intraperitoneal injection of lipopolysaccharide to induce microglial activation, and the rats were killed at different time points. Along with this, primary cultured microglial cells were subjected to lipopolysaccharide treatment, and expression of CNPase was analyzed by real-time reverse transcription PCR and immunofluorescence. Additionally, siRNA transfection was employed to downregulate CNPase in BV-2 cells. Following this, inducible nitric oxide synthase, IL-1β and TNF-α were determined at mRNA and protein levels. Reactive oxygen species and nitric oxide were also assessed by flow cytometry and colorimetric assay, respectively. In parallel to this, CNPase expression in activated microglia was also investigated in adult rats subjected to fluid percussion injury as well as middle cerebral artery occlusion.
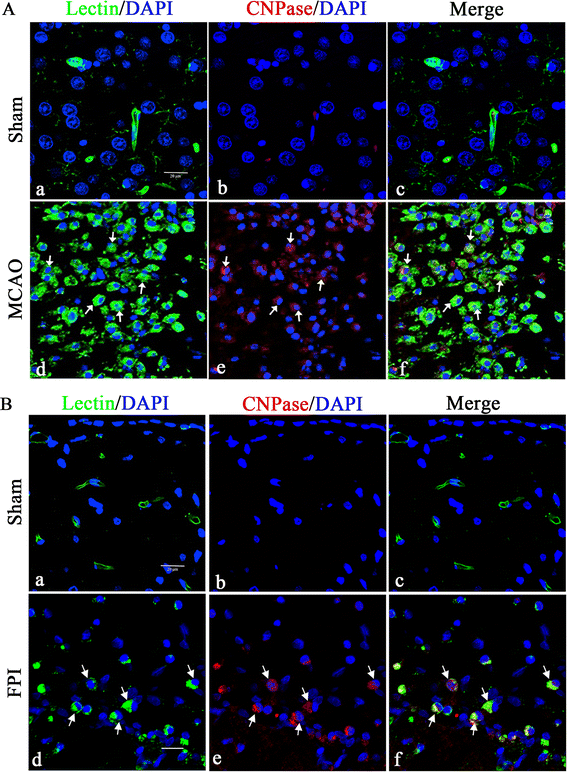
Results: In vivo, CNPase immunofluorescence in activated microglia was markedly enhanced after lipopolysaccharide treatment. A similar feature was observed in the rat brain after fluid percussion injury and middle cerebral artery occlusion. In vitro, CNPase protein and mRNA expression was increased in primary microglia with lipopolysaccharide stimulation. Remarkably, inducible nitric oxide synthase, IL-1β, TNF-α, reactive oxygen species and nitric oxide were significantly upregulated in activated BV-2 cells with CNPase knockdown. siRNA knockdown of CNPase increased microglia migration; on the other hand, microglial cells appeared to be arrested at G1 phase.
Conclusions: The present results have provided the first morphological and molecular evidence that CNPase expression is increased in activated microglia. CNPase knockdown resulted in increased expression of various inflammatory mediators. It is concluded that CNPase may play an important role as a putative anti-inflammatory gene both in normal and injured brain.
Figures






Similar articles
-
Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia.J Neuroinflammation. 2013 Feb 6;10:23. doi: 10.1186/1742-2094-10-23. J Neuroinflammation. 2013. PMID: 23388509 Free PMC article.
-
Expression of 2',3'-cyclic nucleotide 3'-phosphodiesterase in the amoeboid microglial cells in the developing rat brain.Neuroscience. 2006 Oct 13;142(2):333-41. doi: 10.1016/j.neuroscience.2006.06.030. Epub 2006 Jul 28. Neuroscience. 2006. PMID: 16876328
-
Anti-inflammatory effects of Edaravone and Scutellarin in activated microglia in experimentally induced ischemia injury in rats and in BV-2 microglia.BMC Neurosci. 2014 Nov 22;15:125. doi: 10.1186/s12868-014-0125-3. BMC Neurosci. 2014. PMID: 25416145 Free PMC article.
-
Dihydropyrimidinase-like 3 regulates the inflammatory response of activated microglia.Neuroscience. 2013 Dec 3;253:40-54. doi: 10.1016/j.neuroscience.2013.08.023. Epub 2013 Aug 27. Neuroscience. 2013. PMID: 23988434
-
NG2, a member of chondroitin sulfate proteoglycans family mediates the inflammatory response of activated microglia.Neuroscience. 2010 Jan 20;165(2):386-94. doi: 10.1016/j.neuroscience.2009.10.022. Neuroscience. 2010. PMID: 19878709
Cited by
-
The Functions of Mitochondrial 2',3'-Cyclic Nucleotide-3'-Phosphodiesterase and Prospects for Its Future.Int J Mol Sci. 2020 May 1;21(9):3217. doi: 10.3390/ijms21093217. Int J Mol Sci. 2020. PMID: 32370072 Free PMC article. Review.
-
Brain and Serum Membrane Vesicle (Exosome) Profiles in Experimental Alcohol-Related Brain Degeneration: Forging the Path to Non-Invasive Liquid Biopsy Diagnostics.J Mol Pathol (Basel). 2024 Sep;5(3):360-384. doi: 10.3390/jmp5030025. Epub 2024 Sep 10. J Mol Pathol (Basel). 2024. PMID: 39931524 Free PMC article.
-
Exploring synergistic effects: Atorvastatin and electrical stimulation in spinal cord injury therapy.IBRO Neurosci Rep. 2025 Feb 25;18:389-399. doi: 10.1016/j.ibneur.2025.02.012. eCollection 2025 Jun. IBRO Neurosci Rep. 2025. PMID: 40124115 Free PMC article.
-
Spatiotemporal patterns of gene expression around implanted silicon electrode arrays.J Neural Eng. 2021 Apr 27;18(4):10.1088/1741-2552/abf2e6. doi: 10.1088/1741-2552/abf2e6. J Neural Eng. 2021. PMID: 33780909 Free PMC article.
-
Neurotoxocarosis alters myelin protein gene transcription and expression.Parasitol Res. 2015 Jun;114(6):2175-86. doi: 10.1007/s00436-015-4407-1. Epub 2015 Mar 17. Parasitol Res. 2015. PMID: 25773181
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

