Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation
- PMID: 25149517
- PMCID: PMC4249044
- DOI: 10.1128/AEM.01789-14
Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation
Abstract
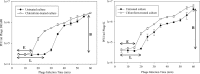
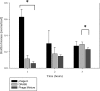
Biofilms are major causes of impairment of wound healing and patient morbidity. One of the most common and aggressive wound pathogens is Staphylococcus aureus, displaying a large repertoire of virulence factors and commonly reduced susceptibility to antibiotics, such as the spread of methicillin-resistant S. aureus (MRSA). Bacteriophages are obligate parasites of bacteria. They multiply intracellularly and lyse their bacterial host, releasing their progeny. We isolated a novel phage, DRA88, which has a broad host range among S. aureus bacteria. Morphologically, the phage belongs to the Myoviridae family and comprises a large double-stranded DNA (dsDNA) genome of 141,907 bp. DRA88 was mixed with phage K to produce a high-titer mixture that showed strong lytic activity against a wide range of S. aureus isolates, including representatives of the major international MRSA clones and coagulase-negative Staphylococcus. Its efficacy was assessed both in planktonic cultures and when treating established biofilms produced by three different biofilm-producing S. aureus isolates. A significant reduction of biofilm biomass over 48 h of treatment was recorded in all cases. The phage mixture may form the basis of an effective treatment for infections caused by S. aureus biofilms.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures






References
-
- Plata K, Rosato AE, Wegrzyn G. 2009. Staphylococcus aureus as an infectious agent: overview of biochemistry and molecular genetics of its pathogenicity. Acta Biochim. Pol. 56:597–612. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

