Preservation of genetic and regulatory robustness in ancient gene duplicates of Saccharomyces cerevisiae
- PMID: 25149527
- PMCID: PMC4216924
- DOI: 10.1101/gr.176792.114
Preservation of genetic and regulatory robustness in ancient gene duplicates of Saccharomyces cerevisiae
Abstract
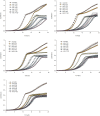
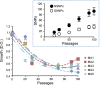
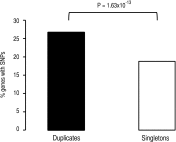
Biological systems remain robust against certain genetic and environmental challenges. Robustness allows the exploration of ecological adaptations. It is unclear what factors contribute to increasing robustness. Gene duplication has been considered to increase genetic robustness through functional redundancy, accelerating the evolution of novel functions. However, recent findings have questioned the link between duplication and robustness. In particular, it remains elusive whether ancient duplicates still bear potential for innovation through preserved redundancy and robustness. Here we have investigated this question by evolving the yeast Saccharomyces cerevisiae for 2200 generations under conditions allowing the accumulation of deleterious mutations, and we put mechanisms of mutational robustness to a test. S. cerevisiae declined in fitness along the evolution experiment, but this decline decelerated in later passages, suggesting functional compensation of mutated genes. We resequenced 28 genomes from experimentally evolved S. cerevisiae lines and found more mutations in duplicates--mainly small-scale duplicates--than in singletons. Genetically interacting duplicates evolved similarly and fixed more amino acid-replacing mutations than expected. Regulatory robustness of the duplicates was supported by a larger enrichment for mutations at the promoters of duplicates than at those of singletons. Analyses of yeast gene expression conditions showed a larger variation in the duplicates' expression than that of singletons under a range of stress conditions, sparking the idea that regulatory robustness allowed a wider range of phenotypic responses to environmental stresses, hence faster adaptations. Our data support the persistence of genetic and regulatory robustness in ancient duplicates and its role in adaptations to stresses.
© 2014 Keane et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


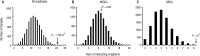

 . Duplicates with larger expression plasticity are colored in red; squares are colored in blue that becomes lighter as the difference in expression decreases between the duplicates; and light yellow indicates that the corresponding information is not sufficiently large to perform statistical tests.
. Duplicates with larger expression plasticity are colored in red; squares are colored in blue that becomes lighter as the difference in expression decreases between the duplicates; and light yellow indicates that the corresponding information is not sufficiently large to perform statistical tests.References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
