Systemic miRNA-7 delivery inhibits tumor angiogenesis and growth in murine xenograft glioblastoma
- PMID: 25149532
- PMCID: PMC4196156
- DOI: 10.18632/oncotarget.2235
Systemic miRNA-7 delivery inhibits tumor angiogenesis and growth in murine xenograft glioblastoma
Abstract
Tumor-angiogenesis is the multi-factorial process of sprouting of endothelial cells (EC) into micro-vessels to provide tumor cells with nutrients and oxygen. To explore miRNAs as therapeutic angiogenesis-inhibitors, we performed a functional screen to identify miRNAs that are able to decrease EC viability. We identified miRNA-7 (miR-7) as a potent negative regulator of angiogenesis. Introduction of miR-7 in EC resulted in strongly reduced cell viability, tube formation, sprouting and migration. Application of miR-7 in the chick chorioallantoic membrane assay led to a profound reduction of vascularization, similar to anti-angiogenic drug sunitinib. Local administration of miR-7 in an in vivo murine neuroblastoma tumor model significantly inhibited angiogenesis and tumor growth. Finally, systemic administration of miR-7 using a novel integrin-targeted biodegradable polymeric nanoparticles that targets both EC and tumor cells, strongly reduced angiogenesis and tumor proliferation in mice with human glioblastoma xenografts. Transcriptome analysis of miR-7 transfected EC in combination with in silico target prediction resulted in the identification of OGT as novel target gene of miR-7. Our study provides a comprehensive validation of miR-7 as novel anti-angiogenic therapeutic miRNA that can be systemically delivered to both EC and tumor cells and offers promise for miR-7 as novel anti-tumor therapeutic.
Figures




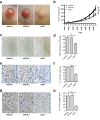
References
-
- Skalak TC. Angiogenesis and microvascular remodeling: a brief history and future roadmap. Microcirculation. 2005;12(1):47–58. - PubMed
-
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267(16):10931–10934. - PubMed
-
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. - PubMed
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

