Characterizing, controlling and eliminating residual malaria transmission
- PMID: 25149656
- PMCID: PMC4159526
- DOI: 10.1186/1475-2875-13-330
Characterizing, controlling and eliminating residual malaria transmission
Abstract
Long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) interventions can reduce malaria transmission by targeting mosquitoes when they feed upon sleeping humans and/or rest inside houses, livestock shelters or other man-made structures. However, many malaria vector species can maintain robust transmission, despite high coverage of LLINs/IRS containing insecticides to which they are physiologically fully susceptible, because they exhibit one or more behaviours that define the biological limits of achievable impact with these interventions: (1) Natural or insecticide-induced avoidance of contact with treated surfaces within houses and early exit from them, thus minimizing exposure hazard of vectors which feed indoors upon humans; (2) Feeding upon humans when they are active and unprotected outdoors, thereby attenuating personal protection and any consequent community-wide suppression of transmission; (3) Feeding upon animals, thus minimizing contact with insecticides targeted at humans or houses; (4) Resting outdoors, away from insecticide-treated surfaces of nets, walls and roofs. Residual malaria transmission is, therefore, defined as all forms of transmission that can persist after achieving full universal coverage with effective LLINs and/or IRS containing active ingredients to which local vector populations are fully susceptible. Residual transmission is sufficiently intense across most of the tropics to render malaria elimination infeasible without new or improved vector control methods. Many novel or improved vector control strategies to address residual transmission are emerging that either: (1) Enhance control of adult vectors that enter houses to feed and/or rest by killing, repelling or excluding them; (2) Kill or repel adult mosquitoes when they attack people outdoors; (3) Kill adult mosquitoes when they attack livestock; (4) Kill adult mosquitoes when they feed upon sugar or; (5) Kill immature mosquitoes in aquatic habitats. To date, none of these options has sufficient supporting evidence to justify full-scale programmatic implementation. Concerted investment in their rigorous selection, development and evaluation is required over the coming decade to enable control and, ultimately, elimination of residual malaria transmission. In the meantime, national programmes may assess options for addressing residual transmission under programmatic conditions through pilot studies with strong monitoring, evaluation and operational research components, similar to the Onchocerciasis Control Programme.
Figures

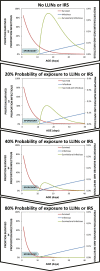

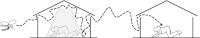






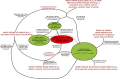
 ; (3) the mortality probability (μ) of mosquitoes utilizing covered forms of that resource subset; where human blood is the targeted resource, (4) the personal protection (ρ) afforded as a result of repellence, irritance or physical deterrence (Δ) combined with fast-acting toxicity that occurs before the mosquito can feed (μ
pre); and (5) the proportion of exposure that would otherwise occur when that intervention is used
; (3) the mortality probability (μ) of mosquitoes utilizing covered forms of that resource subset; where human blood is the targeted resource, (4) the personal protection (ρ) afforded as a result of repellence, irritance or physical deterrence (Δ) combined with fast-acting toxicity that occurs before the mosquito can feed (μ
pre); and (5) the proportion of exposure that would otherwise occur when that intervention is used
 . For all parameters described, values approaching or exceeding one are considered high and values approaching zero are considered low. The subscripts h, l and i refer to the subsets human, livestock and indoors.
. For all parameters described, values approaching or exceeding one are considered high and values approaching zero are considered low. The subscripts h, l and i refer to the subsets human, livestock and indoors.References
-
- Service MW, Townson H. The Anopheles Vector. In: Gilles HM, Warrell DA, editors. Essential Malariology. Fourth. London: Arnold; 2002. pp. 59–84.
-
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

