HPV catch-up vaccination of young women: a systematic review and meta-analysis
- PMID: 25149765
- PMCID: PMC4159543
- DOI: 10.1186/1471-2458-14-867
HPV catch-up vaccination of young women: a systematic review and meta-analysis
Abstract
Background: While prophylactic human papilloma virus (HPV) vaccination is considered effective in young girls, it is unclear whether a catch-up vaccination of older girls would be beneficial. We, therefore, aimed to examine the potential health impact of a HPV catch-up vaccination of girls who were too old at the time of vaccine introduction, hence aged 16 and older.
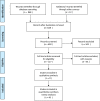
Methods: We systematically searched the literature for randomized clinical trials (RCTs) that examined the effect of HPV vaccines on overall mortality, cancer mortality and incidence, high-grade cervical intraepithelial neoplasia grade 2 and higher (CIN2+), vulvar intraepithelial neoplasia (VIN) and vaginal intraepithelial neoplasia (VaIN) grade 2 and higher lesions (VIN2+ and VaIN2+, respectively) genital warts (condyloma). We considered all lesions and those associated with HPV type(s) included in the vaccines. RCTs reporting on serious adverse events were also eligible. Selected publications were assessed for potential risk of bias, and we ascertained the overall quality of the evidence for each outcome using Grading of Recommendations Assessment, Development and Evaluation (GRADE). Meta-analyses were performed, assuming both random and fixed effects, to estimate risk ratios (RR) and corresponding 95% confidence intervals (CI), using intention-to-treat and per-protocol populations.
Results: We included 46 publications reporting on 13 RCTs. Most of the RCTs had a maximum follow-up period of four years. We identified no RCT reporting on the effect of HPV catch vaccination on overall and cancer related mortality, and on cervical cancer incidence. We found a borderline protective effect of a HPV catch-up vaccination on all CIN2+, with a pooled RR of 0.80 (95% CI: 0.62-1.02) for a follow-up period of 4 years. A HPV catch-up vaccination was associated with a reduction in VIN2+ and VaIN2+ lesions, and condyloma. No difference in risk of serious adverse events was seen in vaccinated participants versus unvaccinated women (pooled RR of 0.99 (0.91-1.08)).
Conclusions: This systematic review indicates that a HPV catch-up vaccination could be beneficial, however the long-term effect of such a vaccination, and its effect on cervical cancer incidence and mortality is still unclear.
Figures





References
-
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, on behalf of the WHO International Agency for Research on Cancer Monograph Working Group Biological agents. volume 100 B. a review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt B):1–441.
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2458/14/867/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

