Extracorporeal photopheresis as second-line treatment for acute graft-versus-host disease: impact on six-month freedom from treatment failure
- PMID: 25150260
- PMCID: PMC4222474
- DOI: 10.3324/haematol.2014.108217
Extracorporeal photopheresis as second-line treatment for acute graft-versus-host disease: impact on six-month freedom from treatment failure
Abstract
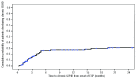
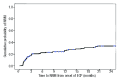
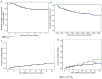
Second-line therapy for corticosteroid-refractory or -dependent acute graft-versus-host disease remains ill-defined, due to limited efficacy of drugs and evolving clinical trial endpoints. Six-month freedom from treatment failure has been proposed as a novel clinical trial endpoint and is defined by the absence of death, malignancy relapse/progression, or addition of a next line of systemic immunosuppressive therapy within 6 months of intervention and prior to diagnosis of chronic graft-versus-host disease. We analyzed the 6-month freedom from treatment failure endpoint in 128 patients enrolled from three centers who were treated with extracorporeal photopheresis as second-line therapy for acute graft-versus-host disease. The incidence of 6-month freedom from treatment failure was 77.3% with a 2-year survival rate of 56%. Corticosteroid dose or response status at onset of second-line therapy did not influence outcome. Higher grade of acute graft-versus-host disease (grade 2 versus grades 3-4) at onset of photopheresis predicted for poor outcome as measured by survival (hazard ratio 2.78, P<0.001), non-relapse mortality (hazard ratio 2.78, P=0.001) and 6-month freedom from treatment failure (hazard ratio 3.05, P<0.001). For the 91 patients who achieved 6-month freedom from treatment failure, 1-year, 2-year and 3-year survival rates were 78.9%, 70.8% and 69.5%, respectively. Six-month freedom from treatment failure is a reasonable early surrogate for outcome and should be considered as a clinical trial endpoint. This study demonstrates the durable effect of photopheresis as second-line therapy for corticosteroid-refractory or -dependent acute graft-versus-host disease using 6-month freedom from treatment failure as the primary endpoint.
Copyright© Ferrata Storti Foundation.
Figures
Similar articles
-
Favorable impact of extracorporeal photopheresis in acute and chronic graft versus host disease: Prospective single-center study.J Clin Apher. 2018 Dec;33(6):654-660. doi: 10.1002/jca.21660. Epub 2018 Nov 5. J Clin Apher. 2018. PMID: 30394564
-
Extracorporeal photopheresis as first-line strategy in the treatment of acute graft-versus-host disease after hematopoietic stem cell transplantation: A single-center experience.Cytotherapy. 2020 Aug;22(8):445-449. doi: 10.1016/j.jcyt.2020.03.003. Epub 2020 May 18. Cytotherapy. 2020. PMID: 32434750
-
Extracorporeal photopheresis in the treatment of acute graft-versus-host disease: A multicenter experience.Transfus Apher Sci. 2021 Oct;60(5):103242. doi: 10.1016/j.transci.2021.103242. Epub 2021 Aug 13. Transfus Apher Sci. 2021. PMID: 34420882
-
Extracorporeal photopheresis in acute and chronic graft-versus-host disease.Transfus Apher Sci. 2014 Jun;50(3):349-57. doi: 10.1016/j.transci.2014.04.005. Epub 2014 Apr 13. Transfus Apher Sci. 2014. PMID: 24780392 Review.
-
The role of the extracorporeal photopheresis in the management of the graft-versus-host disease.Transfus Apher Sci. 2012 Apr;46(2):211-9. doi: 10.1016/j.transci.2011.10.018. Epub 2011 Nov 27. Transfus Apher Sci. 2012. PMID: 22123355 Review.
Cited by
-
Extracorporeal photopheresis versus alternative treatment for chronic graft-versus-host disease after haematopoietic stem cell transplantation in paediatric patients.Cochrane Database Syst Rev. 2015 Dec 15;2015(12):CD009898. doi: 10.1002/14651858.CD009898.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2022 Jun 9;6:CD009898. doi: 10.1002/14651858.CD009898.pub4. PMID: 26666581 Free PMC article. Updated.
-
ECP versus ruxolitinib in steroid-refractory acute GVHD - a retrospective study by the EBMT transplant complications working party.Front Immunol. 2023 Dec 11;14:1283034. doi: 10.3389/fimmu.2023.1283034. eCollection 2023. Front Immunol. 2023. PMID: 38149251 Free PMC article.
-
Effective Extracorporeal Photopheresis of Patients with Transplantation Induced Acute Intestinal GvHD and Bronchiolitis Obliterans Syndrome.Biomedicines. 2022 Aug 4;10(8):1887. doi: 10.3390/biomedicines10081887. Biomedicines. 2022. PMID: 36009436 Free PMC article.
-
The Role of Extracorporeal Photopheresis in the Management of Graft Versus Host Disease: Narrative Review.Immunotargets Ther. 2024 Apr 26;13:235-246. doi: 10.2147/ITT.S457366. eCollection 2024. Immunotargets Ther. 2024. PMID: 38689598 Free PMC article. Review.
-
Extracorporeal photopheresis for treatment of adults and children with acute GVHD: UK consensus statement and review of published literature.Bone Marrow Transplant. 2014 Oct;49(10):1251-8. doi: 10.1038/bmt.2014.106. Epub 2014 Jun 2. Bone Marrow Transplant. 2014. PMID: 24887389 Review.
References
-
- Murata M, Nakasone H, Kanda J, Nakane T, Furukawa T, Fukuda T, et al. Clinical factors predicting the response of acute graft-versus-host disease to corticosteroid therapy: an analysis from the GVHD working group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2013;19(8):1183–9. - PubMed
-
- MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115(26):5412–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

