Adipocytes in both brown and white adipose tissue of adult mice are functionally connected via gap junctions: implications for Chagas disease
- PMID: 25150689
- PMCID: PMC4353925
- DOI: 10.1016/j.micinf.2014.08.006
Adipocytes in both brown and white adipose tissue of adult mice are functionally connected via gap junctions: implications for Chagas disease
Abstract
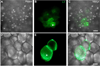
Adipose tissue serves as a host reservoir for the protozoan Trypanosoma cruzi, the causative organism in Chagas disease. Gap junctions interconnect cells of most tissues, serving to synchronize cell activities including secretion in glandular tissue, and we have previously demonstrated that gap junctions are altered in various tissues and cells infected with T. cruzi. Herein, we examined the gap junction protein connexin 43 (Cx43) expression in infected adipose tissues. Adipose tissue is the largest endocrine organ of the body and is also involved in other physiological functions. In mammals, it is primarily composed of white adipocytes. Although gap junctions are a prominent feature of brown adipocytes, they have not been explored extensively in white adipocytes, especially in the setting of infection. Thus, we examined functional coupling in both white and brown adipocytes in mice. Injection of electrical current or the dye Lucifer Yellow into adipocytes within fat tissue spread to adjacent cells, which was reduced by treatment with agents known to block gap junctions. Moreover, Cx43 was detected in both brown and white fat tissue. At thirty and ninety days post-infection, Cx43 was downregulated in brown adipocytes and upregulated in white adipocytes. Gap junction-mediated intercellular communication likely contributes to hormone secretion and other functions in white adipose tissue and to nonshivering thermogenesis in brown fat, and modulation of the coupling by T. cruzi infection is expected to impact these functions.
Keywords: Adipose tissue; Chagas disease; Connexin43; Gap junctions; Trypanosoma cruzi.
Copyright © 2014. Published by Elsevier Masson SAS.
Figures
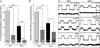



References
-
- Michon L, Nlend Nlend R, Bavamian S, Bischoff L, Boucard N, Caille D, Cancela J, Charollais A, Charpantier E, Klee P, Peyrou M, Populaire C, Zulianello L, Meda P. Involvement of gap junctional communication in secretion. Biochim Biophys Acta. 2005;1719:82–101. - PubMed
-
- Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. - PubMed
-
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

