Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study
- PMID: 25150798
- PMCID: PMC4174348
- DOI: 10.1016/S1470-2045(14)70335-3
Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study
Abstract
Background: Ibrutinib, an orally administered covalent inhibitor of Bruton's tyrosine kinase (BTK), is an effective treatment for relapsed chronic lymphocytic leukaemia (CLL). We investigated the activity and safety of the combination of ibrutinib with the monoclonal antibody rituximab in patients with high-risk CLL.
Methods: In this single-arm phase 2 study, we enrolled adult patients with high-risk CLL at the MD Anderson Cancer Center (Houston, TX, USA). All enrolled participants had high-risk cytogenetic abnormalities (deletion 17p, TP53 mutation, or deletion 11q) or a short progression-free survival (PFS <36 months) after previous first-line chemoimmunotherapy. Patients with symptomatic disease requiring therapy received 28-day cycles of once-daily ibrutinib 420 mg together with rituximab (375 mg/m(2), intravenously, every week during cycle 1, then once per cycle until cycle 6), followed by continuous daily single-agent ibrutinib 420 mg until disease progression or until toxicities or complications precluded further treatment. The primary endpoint was progression-free survival in the intention-to-treat population. This study is registered with ClinicalTrials.gov number NCT01520519, and is no longer accruing patients.
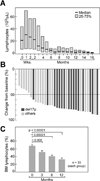
Findings: Between Feb 28, 2012, and Sept 11, 2012, we enrolled 40 patients with CLL with high-risk disease features, 20 of whom had deletion 17p (del[17p]) or TP53 mutations (16 previously treated, four untreated), 13 had relapsed CLL with deletion 11q (del[11q]), and seven a PFS less than 36 months after first-line chemoimmunotherapy. 18-month PFS in all patients was 78·0% (95% CI 60·6-88·5), whereas in those with a del(17p) or TP53 mutation it was 72·4% (45·6-87·6) Toxicity was mainly mild to moderate in severity (grade 1-2). Diarrhoea occurred in ten (25%) patients (grade 1 in nine patients and grade 2 in one), bleeding events in 14 (33%) patients (eight grade 1 and five grade 2), nausea or vomiting in 15 patients (38%) (ten grade 1 and five grade 2), and fatigue in seven (18%) patients (four grade 1 and three grade 2). Five patients (13%) had grade 3 infections (two lung infections, one upper respiratory tract infection, one sepsis, and one mucositis), and no grade 4 or 5 infections occurred. One patient had grade 4 neutropenia.
Interpretation: The encouraging safety and activity of ibrutinib and rituximab in this population of patients with high-risk CLL merits further investigation of this combination.
Funding: Pharmacyclics Inc, Cancer Prevention and Research Institute of Texas, Leukemia and Lymphoma Society, National Cancer Institute, MD Anderson Cancer Center.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
JAB and SOB received research funding from Pharmacyclics, Inc.; JAB is a consultant for Janssen Pharmaceuticals, Inc.. All other authors declare that they have no conflict of interest.
Figures

Comment in
-
Ibrutinib: better combined with other drugs?Lancet Oncol. 2014 Sep;15(10):1043-4. doi: 10.1016/S1470-2045(14)70388-2. Epub 2014 Aug 20. Lancet Oncol. 2014. PMID: 25150799 No abstract available.
References
-
- Hallek M. Signaling the end of chronic lymphocytic leukemia: new frontline treatment strategies. Blood. 2013;122(23):3723–3734. - PubMed
-
- Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. The New England journal of medicine. 2014 - PubMed
-
- Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. The New England journal of medicine. 2000;343(26):1910–1916. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

