Comparative assessment of the bacterial communities associated with Aedes aegypti larvae and water from domestic water storage containers
- PMID: 25151134
- PMCID: PMC4156648
- DOI: 10.1186/1756-3305-7-391
Comparative assessment of the bacterial communities associated with Aedes aegypti larvae and water from domestic water storage containers
Abstract
Background: Domestic water storage containers constitute major Aedes aegypti breeding sites. We present for the first time a comparative analysis of the bacterial communities associated with Ae. aegypti larvae and water from domestic water containers.
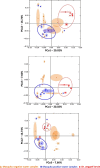
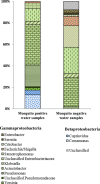
Methods: The 16S rRNA-temporal temperature gradient gel electrophoresis (TTGE) was used to identify and compare bacterial communities in fourth-instar Ae. aegypti larvae and water from larvae positive and negative domestic containers in a rural village in northeastern Thailand. Water samples were cultured for enteric bacteria in addition to TTGE. Sequences obtained from TTGE and bacterial cultures were clustered into operational taxonomic units (OTUs) for analyses.
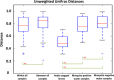
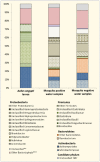
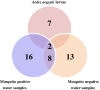
Results: Significantly lower OTU abundance was found in fourth-instar Ae. aegypti larvae compared to mosquito positive water samples. There was no significant difference in OTU abundance between larvae and mosquito negative water samples or between mosquito positive and negative water samples. Larval samples had significantly different OTU diversity compared to mosquito positive and negative water samples, with no significant difference between mosquito positive and negative water samples. The TTGE identified 24 bacterial taxa, belonging to the phyla Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes and TM7 (candidate phylum). Seven of these taxa were identified in larval samples, 16 in mosquito positive and 13 in mosquito negative water samples. Only two taxa, belonging to the phyla Firmicutes and Actinobacteria, were common to both larvae and water samples. Bacilli was the most abundant bacterial class identified from Ae. aegypti larvae, Gammaproteobacteria from mosquito positive water samples, and Flavobacteria from mosquito negative water samples. Enteric bacteria belonging to the class Gammaproteobacteria were sparsely represented in TTGE, but were isolated from both mosquito positive and negative water samples by selective culture.
Conclusions: Few bacteria from water samples were identified in fourth-instar Ae. aegypti larvae, suggesting that established larval bacteria, most likely acquired at earlier stages of development, control the larval microbiota. Further studies at all larval stages are needed to fully understand the dynamics involved. Isolation of enteric bacteria from water samples supports earlier outcomes of E. coli contamination in Ae. aegypti infested domestic containers, suggesting the need to further explore the role of enteric bacteria in Ae. aegypti infestation.
Figures

References
-
- Chareonviriyaphap T, Akratanakul P, Nettanomsak S, Huntamai S. Larval habitats and distribution patterns of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse), in Thailand. Southeast Asian J Trop Med Public Health. 2003;34(3):529–535. - PubMed
-
- Aziz AT, Dieng H, Ahmad AH, Mahyoub JA, Turkistani AM, Mesed H, Koshike S, Satho T, Salmah MRC, Ahmad H, Zuharah WF, Ramli AS, Miake F. Household survey of container–breeding mosquitoes and climatic factors influencing the prevalence of Aedes aegypti (Diptera: Culicidae) in Makkah City, Saudi Arabia. Asian Pac J Trop Biomed. 2012;2(11):849–857. doi: 10.1016/S2221-1691(12)60242-1. - DOI - PMC - PubMed
-
- Hiscox A, Kaye A, Vongphayloth K, Banks I, Piffer M, Khammanithong P, Sananikhom P, Kaul S, Hill N, Lindsay SW, Brey PT. Risk factors for the presence of Aedes aegypti and Aedes albopictus in domestic water-holding containers in areas impacted by the Nam Theun 2 hydroelectric project. Laos Am J Trop Med Hyg. 2013;88(6):1070–1078. doi: 10.4269/ajtmh.12-0623. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

