A microbial biomanufacturing platform for natural and semisynthetic opioids
- PMID: 25151135
- PMCID: PMC4167936
- DOI: 10.1038/nchembio.1613
A microbial biomanufacturing platform for natural and semisynthetic opioids
Abstract
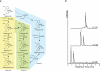
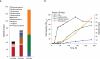
Opiates and related molecules are medically essential, but their production via field cultivation of opium poppy Papaver somniferum leads to supply inefficiencies and insecurity. As an alternative production strategy, we developed baker's yeast Saccharomyces cerevisiae as a microbial host for the transformation of opiates. Yeast strains engineered to express heterologous genes from P. somniferum and bacterium Pseudomonas putida M10 convert thebaine to codeine, morphine, hydromorphone, hydrocodone and oxycodone. We discovered a new biosynthetic branch to neopine and neomorphine, which diverted pathway flux from morphine and other target products. We optimized strain titer and specificity by titrating gene copy number, enhancing cosubstrate supply, applying a spatial engineering strategy and performing high-density fermentation, which resulted in total opioid titers up to 131 mg/l. This work is an important step toward total biosynthesis of valuable benzylisoquinoline alkaloid drug molecules and demonstrates the potential for developing a sustainable and secure yeast biomanufacturing platform for opioids.
Figures
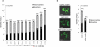

References
-
- Bernáth J. Poppy: the genus Papaver. Harwood Academic Publishers; Amsterdam, The Netherlands: 1998.
-
- International Narcotics Control Board Narcotic Drugs . Estimated World Requirements for 2013, Statistics for 2011. New York: 2012.
-
- PDR Network Physicians' Desk Reference, Edn. 66. Montvale, NJ: 2012.
-
- Hagel JM, Facchini PJ. Benzylisoquinoline alkaloid metabolism: a century of discovery and a brave new world. Plant Cell Physiol. 2013;54:647–672. - PubMed
-
- Rinner U, Hudlicky T. Synthesis of morphine alkaloids and derivatives. Top Curr Chem. 2012;309:33–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

