Image familiarization sharpens response dynamics of neurons in inferotemporal cortex
- PMID: 25151263
- PMCID: PMC4613775
- DOI: 10.1038/nn.3794
Image familiarization sharpens response dynamics of neurons in inferotemporal cortex
Abstract
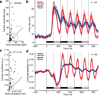
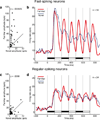
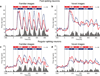
Repeated viewing of an image over days and weeks induces a marked reduction in the strength with which neurons in monkey inferotemporal cortex respond to it. The processing advantage that attaches to this reduction is unknown. One possibility is that truncation of the response to a familiar image leaves neurons in a state of readiness to respond to ensuing images and thereby enhances their ability to track rapidly changing displays. We explored this possibility by assessing neuronal responses to familiar and novel images in rapid serial visual displays. Inferotemporal neurons responded more strongly to familiar than to novel images in such displays. The effect was stronger among putative inhibitory neurons than among putative excitatory neurons. A comparable effect occurred at the level of the scalp potential in humans. We conclude that long-term familiarization sharpens the response dynamics of neurons in both monkey and human extrastriate visual cortex.
Figures



References
-
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. MIT Press Cambridge, MA; 1982. pp. 549–586.
-
- Sobotka S, Ringo J. Investigation of long-term recognition and association memory in unit responses from inferotemporal cortex. Experimental Brain Research. 1993;96:28–38. - PubMed
-
- Fahy FL, Riches IP, Brown MW. Neuronal activity related to visual recognition memory: long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Experimental Brain Research. 1993;96:457–472. - PubMed
-
- Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37:657–676. - PubMed
-
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Experience-dependent sharpening of visual shape selectivity in inferior temporal cortex. Cerebral Cortex. 2006;16:1631–1644. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

