B cell homeostasis and follicle confines are governed by fibroblastic reticular cells
- PMID: 25151489
- PMCID: PMC4205585
- DOI: 10.1038/ni.2965
B cell homeostasis and follicle confines are governed by fibroblastic reticular cells
Abstract
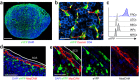
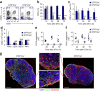
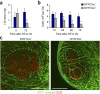
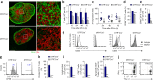
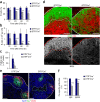
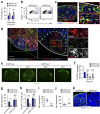
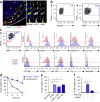
Fibroblastic reticular cells (FRCs) are known to inhabit T cell-rich areas of lymphoid organs, where they function to facilitate interactions between T cells and dendritic cells. However, in vivo manipulation of FRCs has been limited by a dearth of genetic tools that target this lineage. Here, using a mouse model to conditionally ablate FRCs, we demonstrated their indispensable role in antiviral T cell responses. Unexpectedly, loss of FRCs also attenuated humoral immunity due to impaired B cell viability and follicular organization. Follicle-resident FRCs established a favorable niche for B lymphocytes via production of the cytokine BAFF. Thus, our study indicates that adaptive immunity requires an intact FRC network and identifies a subset of FRCs that control B cell homeostasis and follicle identity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Turley SJ, Fletcher AL, Elpek KG. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat. Rev. Immunol. 2010;10:813–825. - PubMed
-
- Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 2000;192:1425–1440. - PMC - PubMed
-
- Sixt M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

