Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice
- PMID: 25151491
- PMCID: PMC4169290
- DOI: 10.1038/ni.2967
Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice
Erratum in
- Nat Immunol. 2014 Nov;15(11):1090
Abstract
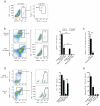
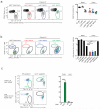
The paradigm that macrophages that reside in steady-state tissues are derived from embryonic precursors has never been investigated in the intestine, which contains the largest pool of macrophages. Using fate-mapping models and monocytopenic mice, together with bone marrow chimera and parabiotic models, we found that embryonic precursor cells seeded the intestinal mucosa and demonstrated extensive in situ proliferation during the neonatal period. However, these cells did not persist in the intestine of adult mice. Instead, they were replaced around the time of weaning by the chemokine receptor CCR2-dependent influx of Ly6C(hi) monocytes that differentiated locally into mature, anti-inflammatory macrophages. This process was driven largely by the microbiota and had to be continued throughout adult life to maintain a normal intestinal macrophage pool.
Figures




References
-
- Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science (New York, N.Y. 2012;336:86–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

