A new tool to guide halofunctionalization reactions: the halenium affinity (HalA) scale
- PMID: 25152188
- PMCID: PMC4183602
- DOI: 10.1021/ja506889c
A new tool to guide halofunctionalization reactions: the halenium affinity (HalA) scale
Abstract
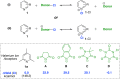
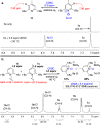
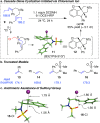
We introduce a previously unexplored parameter-halenium affinity (HalA)- as a quantitative descriptor of the bond strengths of various functional groups to halenium ions. The HalA scale ranks potential halenium ion acceptors based on their ability to stabilize a "free halenium ion". Alkenes in particular but other Lewis bases as well, such as amines, amides, carbonyls, and ether oxygen atoms, etc., have been classified on the HalA scale. This indirect approach enables a rapid and straightforward prediction of chemoselectivity for systems involved in halofunctionalization reactions that have multiple nucleophilic sites. The influences of subtle electronic and steric variations, as well as the less predictable anchimeric and stereoelectronic effects, are intrinsically accounted for by HalA computations, providing quantitative assessments beyond simple "chemical intuition". This combined theoretical-experimental approach offers an expeditious means of predicting and identifying unprecedented reactions.
Figures






References
-
- Bose A.; Mal P. Tetrahedron Lett. 2014, 55, 2154.
- Chen J.; Zhou L. Synthesis 2014, 46, 586.
- de Mattos M. C. S. Curr. Org. Synth. 2013, 10, 819.
- Galligan M. J.; Akula R.; Ibrahim H. Org. Lett. 2014, 16, 600. - PubMed
- Getrey L.; Krieg T.; Hollmann F.; Schrader J.; Holtmann D. Green Chem. 2014, 16, 1104.
- Kamei T.; Ishibashi A.; Shimada T. Tetrahedron Lett. 2014, 55, 4245.
- Stodulski M.; Goetzinger A.; Kohlhepp S. V.; Gulder T. Chem. Commun. 2014, 50, 3435. - PubMed
-
- Hiegel G. A.; Nalbandy M. Synth. Commun. 1992, 22, 1589.
- van Summeren R. P.; Romaniuk A.; Ijpeij E. G.; Alsters P. L. Catal. Sci. Technol. 2012, 2, 2052.
-
- Hasty S. J.; Demchenko A. V. Chem. Heterocycl. Compd. 2012, 48, 220. - PMC - PubMed
- Girard N.; Rousseau C.; Martin O. R. Tetrahedron Lett. 2003, 44, 8971.
- Fraser-Reid B.; Lopez C. J.; Gammon D. W.; Sels B. F. In Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance; Demchenko A. V., Ed.; Wiley: 2008; p 381.
-
- In Halogen Bonding: Fundamentals and Applications; Metrangolo P., Resnati G., Eds.; Springer-Verlag Berlin: Berlin, 2008; Vol. 126, p 1.
- Chen G.; Ma S. Angew. Chem., Int. Ed. 2010, 49, 8306. - PubMed
- Castellanos A.; Fletcher S. P. Chem.—Eur. J. 2011, 17, 5766. - PubMed
- Denmark S. E.; Kuester W. E.; Burk M. T. Angew. Chem., Int. Ed. 2012, 51, 10938. - PMC - PubMed
- Hennecke U. Chem.—Asian J. 2012, 7, 456. - PubMed
- Mendoza A.; Fananas F. J.; Rodriguez F. Curr. Org. Synth. 2013, 10, 384.
- Murai K.; Fujioka H. Heterocycles 2013, 87, 763.
- Tan C. K.; Yeung Y.-Y. Chem. Commun. 2013, 49, 7985. - PubMed
- Chen J.; Zhou L. Synthesis 2014, 46, 586.
- Zheng S.; Schienebeck C. M.; Zhang W.; Wang H.-Y.; Tang W. Asian J. Org. Chem. 2014, 3, 366.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

