Nucleic Acid Nanostructures: Bottom-Up Control of Geometry on the Nanoscale
- PMID: 25152542
- PMCID: PMC4141725
- DOI: 10.1088/0034-4885/68/1/R05
Nucleic Acid Nanostructures: Bottom-Up Control of Geometry on the Nanoscale
Abstract
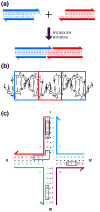
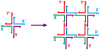
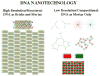
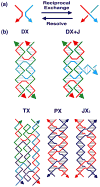
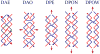
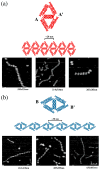
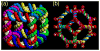
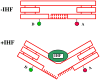
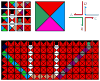
DNA may seem an unlikely molecule from which to build nanostructures, but this is not correct. The specificity of interaction that enables DNA to function so successfully as genetic material also enables its use as a smart molecule for construction on the nanoscale. The key to using DNA for this purpose is the design of stable branched molecules, which expand its ability to interact specifically with other nucleic acid molecules. The same interactions used by genetic engineers can be used to make cohesive interactions with other DNA molecules that lead to a variety of new species. Branched DNA molecules are easy to design, and the can assume a variety of structural motifs. These can be used for purposes both of specific construction, such as polyhedra, and for the assembly of topological targets. A variety of two-dimensional periodic arrays with specific patterns have been made. DNA nanomechanical devices have been built with a series of different triggers, small molecules, nucleic acid molecules and proteins. Recently, progress has been made in self-replication of DNA nano-constructs, and in the scaffolding of other species into DNA arrangements.
Keywords: Branched DNA; DNA devices; DNA periodic arrays; DNA polyhedra; DNA scaffolding; atomic force microscopy; self-assembly; unusual DNA motifs.
Figures



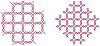


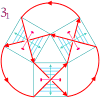
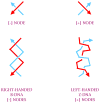
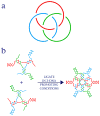





References
-
- Seeman NC. Nucleic acid junctions and lattices. J Theor Biol. 1982;99:237–247. - PubMed
-
- Qiu H, Dewan JC, Seeman NC. A DNA decamer with a sticky end: The crystal structure of d-CGACGATCGT. J Mol Biol. 1997;267:881–898. - PubMed
-
- Seeman NC. At the crossroads of chemistry, biology and materials: structural DNA nanotechnology. Chem & Biol. 2003;10:1151–1159. - PubMed
-
- Holliday R. A mechanism for gene conversion in fungi. Genet Res. 1964;5:282–304. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
