Development of Receptor Targeted Magnetic Iron Oxide Nanoparticles for Efficient Drug Delivery and Tumor Imaging
- PMID: 25152701
- PMCID: PMC4139059
- DOI: 10.1166/jbn.2008.007
Development of Receptor Targeted Magnetic Iron Oxide Nanoparticles for Efficient Drug Delivery and Tumor Imaging
Abstract
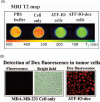
The development of multifunctional nanoparticles that have dual capabilities of tumor imaging and delivering therapeutic agents into tumor cells holds great promises for novel approaches for tumor imaging and therapy. We have engineered urokinase plasminogen activator receptor (uPAR) targeted biodegradable nanoparticles using a size uniform and amphiphilic polymer-coated magnetic iron oxide (IO) nanoparticle conjugated with the amino-terminal fragment (ATF) of urokinase plasminogen activator (uPA), which is a high affinity natural ligand for uPAR. We further developed methods to encapsulate hydrophobic chemotherapeutic drugs into the polymer layer on the IO nanoparticles, making these targeted magnetic resonance imaging (MRI) sensitive nanoparticles drug delivery vehicles. Using a fluorescent drug doxorubicin (Dox) as a model system, we showed that this hydrophobic drug can be efficiently encapsulated into the uPAR-targeted IO nanoparticles. This class of Dox-loaded nanoparticles has a compact size and is stable in pH 7.4 buffer. However, encapsulated Doxcan be released from the nanoparticles at pH 4.0 to 5.0 within 2 hrs. In comparison with the effect of equivalent dosage of free drug or non-targeted IO-Dox nanoparticles, uPAR-targeted IO-Dox nanoparticles deliver higher levels of Dox into breast cancer cells and produce a stronger inhibitory effect on tumor cell growth. Importantly, Dox-loaded IO nanoparticles maintain their T2 MRI contrast effect after being internalized into the tumor cells due to their significant susceptibility effect in the cells, indicating that this drug delivery nanoparticle has the potential to be used as targeted therapeutic imaging probes for monitoring the drug delivery using MRI.
Keywords: Breast Cancer; Doxorubicin; Drug Delivery Nanoparticle; Magnetic Iron Oxide Nanoparticles; Targeted Nanoparticle; uPAR.
Figures




References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J. Clin. 2008;58:71. - PubMed
-
- Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427. - PubMed
-
- Kohler N, Sun C, Fichtenholtz A, Gunn J, Fang C, Zhang M. Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small. 2006;2:785. - PubMed
-
- Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat. Med. 2007;13:372. - PubMed
-
- Chen W, Zhang J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 2006;6:1159. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
