Lipid dynamics at dendritic spines
- PMID: 25152717
- PMCID: PMC4126552
- DOI: 10.3389/fnana.2014.00076
Lipid dynamics at dendritic spines
Abstract
Dynamic changes in the structure and composition of the membrane protrusions forming dendritic spines underlie memory and learning processes. In recent years a great effort has been made to characterize in detail the protein machinery that controls spine plasticity. However, we know much less about the involvement of lipids, despite being major membrane components and structure determinants. Moreover, protein complexes that regulate spine plasticity depend on specific interactions with membrane lipids for proper function and accurate intracellular signaling. In this review we gather information available on the lipid composition at dendritic spine membranes and on its dynamics. We pay particular attention to the influence that spine lipid dynamism has on glutamate receptors, which are key regulators of synaptic plasticity.
Keywords: cholesterol; dendritic spines; glutamate receptors; phosphoinositides; sphingolipids; synaptic plasticity.
Figures

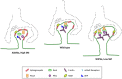
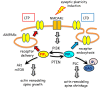
References
-
- Abe M., Makino A., Hullin-Matsuda F., Kamijo K., Ohno-Iwashita Y., Hanada K., et al. (2012). A role for sphingomyelin-rich lipid domains in the accumulation of phosphatidylinositol-4,5-bisphosphate to the cleavage furrow during cytokinesis. Mol. Cell. Biol. 32, 1396–1407 10.1128/MCB.06113-11 - DOI - PMC - PubMed
-
- Arroyo A. I., Camoletto P. G., Morando L., Sassoe-Pognetto M., Giustetto M., Van Veldhoven P. P., et al. (2014). Pharmacological reversion of sphingomyelin-induced dendritic spine anomalies in a Niemann pick disease type a mouse model. EMBO Mol. Med. 6, 398–413 10.1002/emmm.201302649 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

