A Cerebellar Neuroprosthetic System: Computational Architecture and in vivo Test
- PMID: 25152887
- PMCID: PMC4126458
- DOI: 10.3389/fbioe.2014.00014
A Cerebellar Neuroprosthetic System: Computational Architecture and in vivo Test
Abstract
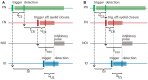
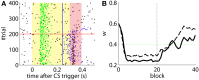
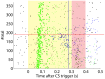
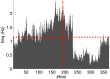
Emulating the input-output functions performed by a brain structure opens the possibility for developing neuroprosthetic systems that replace damaged neuronal circuits. Here, we demonstrate the feasibility of this approach by replacing the cerebellar circuit responsible for the acquisition and extinction of motor memories. Specifically, we show that a rat can undergo acquisition, retention, and extinction of the eye-blink reflex even though the biological circuit responsible for this task has been chemically inactivated via anesthesia. This is achieved by first developing a computational model of the cerebellar microcircuit involved in the acquisition of conditioned reflexes and training it with synthetic data generated based on physiological recordings. Secondly, the cerebellar model is interfaced with the brain of an anesthetized rat, connecting the model's inputs and outputs to afferent and efferent cerebellar structures. As a result, we show that the anesthetized rat, equipped with our neuroprosthetic system, can be classically conditioned to the acquisition of an eye-blink response. However, non-stationarities in the recorded biological signals limit the performance of the cerebellar model. Thus, we introduce an updated cerebellar model and validate it with physiological recordings showing that learning becomes stable and reliable. The resulting system represents an important step toward replacing lost functions of the central nervous system via neuroprosthetics, obtained by integrating a synthetic circuit with the afferent and efferent pathways of a damaged brain region. These results also embody an early example of science-based medicine, where on the one hand the neuroprosthetic system directly validates a theory of cerebellar learning that informed the design of the system, and on the other one it takes a step toward the development of neuro-prostheses that could recover lost learning functions in animals and, in the longer term, humans.
Keywords: association learning; cerebellum; classical conditioning; inferior olive; memory; neuroprosthetics; nucleo-olivary pathway; timing.
Figures












References
-
- Albus J. (1971). A theory of cerebellar function. Math. Biosci. 10, 25–6110.1016/0025-5564(71)90051-4 - DOI
-
- Bamford S., Hogri R., Giovannucci A., Taub A., Herreros I., Verschure P., et al. (2012). A vlsi field-programmable mixed-signal array to perform neural signal processing and neural modeling in a prosthetic system. IEEE Trans. Neural Syst. Rehabil. Eng. 20, 455–46710.1109/TNSRE.2012.2187933 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

