Super-resolution molecular and functional imaging of nanoscale architectures in life and materials science
- PMID: 25152893
- PMCID: PMC4126472
- DOI: 10.3389/fbioe.2014.00020
Super-resolution molecular and functional imaging of nanoscale architectures in life and materials science
Abstract
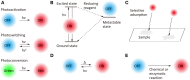
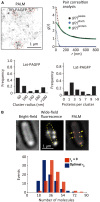
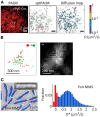
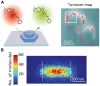
Super-resolution (SR) fluorescence microscopy has been revolutionizing the way in which we investigate the structures, dynamics, and functions of a wide range of nanoscale systems. In this review, I describe the current state of various SR fluorescence microscopy techniques along with the latest developments of fluorophores and labeling for the SR microscopy. I discuss the applications of SR microscopy in the fields of life science and materials science with a special emphasis on quantitative molecular imaging and nanoscale functional imaging. These studies open new opportunities for unraveling the physical, chemical, and optical properties of a wide range of nanoscale architectures together with their nanostructures and will enable the development of new (bio-)nanotechnology.
Keywords: cellular imaging; fluorescence microscopy; nanomaterials; nanostructures; super-resolution.
Figures

Similar articles
-
Super-resolution fluorescent materials: an insight into design and bioimaging applications.Chem Soc Rev. 2016 Aug 22;45(17):4651-67. doi: 10.1039/c5cs00875a. Chem Soc Rev. 2016. PMID: 27296269 Review.
-
Toward Sub-Diffraction Imaging of Single-DNA Molecule Sensors Based on Stochastic Switching Localization Microscopy.Sensors (Basel). 2020 Nov 21;20(22):6667. doi: 10.3390/s20226667. Sensors (Basel). 2020. PMID: 33233370 Free PMC article. Review.
-
When Super-Resolution Localization Microscopy Meets Carbon Nanotubes.Nanomaterials (Basel). 2022 Apr 22;12(9):1433. doi: 10.3390/nano12091433. Nanomaterials (Basel). 2022. PMID: 35564142 Free PMC article. Review.
-
Ultrasensitive Three-Dimensional Orientation Imaging of Single Molecules on Plasmonic Nanohole Arrays Using Second Harmonic Generation.Nano Lett. 2019 Sep 11;19(9):6192-6202. doi: 10.1021/acs.nanolett.9b02239. Epub 2019 Aug 12. Nano Lett. 2019. PMID: 31387355
-
Advancing Wireframe DNA Nanostructures Using Single-Molecule Fluorescence Microscopy Techniques.Acc Chem Res. 2019 Nov 19;52(11):3199-3210. doi: 10.1021/acs.accounts.9b00424. Epub 2019 Nov 1. Acc Chem Res. 2019. PMID: 31675207 Review.
Cited by
-
Engineering a DNA origami mediated multicolour quantum dot platform for sub-diffraction spectral separation imaging.RSC Adv. 2022 Aug 22;12(37):23778-23785. doi: 10.1039/d2ra04316e. eCollection 2022 Aug 22. RSC Adv. 2022. PMID: 36093241 Free PMC article.
-
PAINT-ing Fluorenylmethoxycarbonyl (Fmoc)-Diphenylalanine Hydrogels.Chemistry. 2020 Aug 6;26(44):9869-9873. doi: 10.1002/chem.202001560. Epub 2020 Jun 18. Chemistry. 2020. PMID: 32428285 Free PMC article.
-
Advanced fluorescence microscopy techniques for the life sciences.Glob Cardiol Sci Pract. 2016 Jun 30;2016(2):e201616. doi: 10.21542/gcsp.2016.16. Glob Cardiol Sci Pract. 2016. PMID: 29043264 Free PMC article. Review.
-
Overview about the localization of nanoparticles in tissue and cellular context by different imaging techniques.Beilstein J Nanotechnol. 2015 Jan 23;6:263-80. doi: 10.3762/bjnano.6.25. eCollection 2015. Beilstein J Nanotechnol. 2015. PMID: 25671170 Free PMC article. Review.
-
Study liquid-liquid phase separation with optical microscopy: A methodology review.APL Bioeng. 2023 May 9;7(2):021502. doi: 10.1063/5.0137008. eCollection 2023 Jun. APL Bioeng. 2023. PMID: 37180732 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

