PTK6/BRK is expressed in the normal mammary gland and activated at the plasma membrane in breast tumors
- PMID: 25153721
- PMCID: PMC4171611
- DOI: 10.18632/oncotarget.2153
PTK6/BRK is expressed in the normal mammary gland and activated at the plasma membrane in breast tumors
Abstract
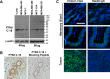
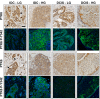
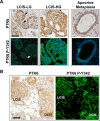
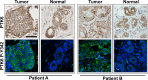
Protein Tyrosine kinase 6 (PTK6/BRK) is overexpressed in the majority of human breast tumors and breast tumor cell lines. It is also expressed in normal epithelial linings of the gastrointestinal tract, skin, and prostate. To date, expression of PTK6 has not been extensively examined in the normal human mammary gland. We detected PTK6 mRNA and protein expression in the immortalized normal MCF-10A human mammary gland epithelial cell line, and examined PTK6 expression and activation in a normal human breast tissue microarray, as well as in human breast tumors. Phosphorylation of tyrosine residue 342 in the PTK6 activation loop corresponds with its activation. Similar to findings in the prostate, we detect nuclear and cytoplasmic PTK6 in normal mammary gland epithelial cells, but no phosphorylation of tyrosine residue 342. However, in human breast tumors, striking PTK6 expression and phosphorylation of tyrosine 342 is observed at the plasma membrane. PTK6 is expressed in the normal human mammary gland, but does not appear to be active and may have kinase-independent functions that are distinct from its cancer promoting activities at the membrane. Understanding consequences of PTK6 activation at the plasma membrane may have implications for developing novel targeted therapies against this kinase.
Conflict of interest statement
The authors have no conflicts of interest to report. This is an original manuscript; data have not been published elsewhere
Figures


References
-
- Serfas MS, Tyner AL. Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol Res. 2003;13(6-10):409–419. - PubMed
-
- Mitchell PJ, Barker KT, Martindale JE, Kamalati T, Lowe PN, Page MJ, Gusterson BA, Crompton MR. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene. 1994;9:2383–2390. - PubMed
-
- Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Research. 2007;67(9):4199–4209. - PubMed
-
- Barker KT, Jackson LE, Crompton MR. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15(7):799–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

