Mineralized gelatin methacrylate-based matrices induce osteogenic differentiation of human induced pluripotent stem cells
- PMID: 25153779
- PMCID: PMC4252258
- DOI: 10.1016/j.actbio.2014.08.010
Mineralized gelatin methacrylate-based matrices induce osteogenic differentiation of human induced pluripotent stem cells
Abstract
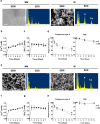
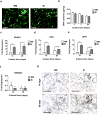
Human induced pluripotent stem cells (hiPSC) are a promising cell source with pluripotency and self-renewal properties. Design of simple and robust biomaterials with an innate ability to induce lineage-specificity of hiPSC is desirable to realize their application in regenerative medicine. In this study, the potential of biomaterials containing calcium phosphate minerals to induce osteogenic differentiation of hiPSC was investigated. hiPSC cultured using mineralized gelatin methacrylate-based matrices underwent osteogenic differentiation ex vivo, in both two-dimensional and three-dimensional cultures, in growth medium devoid of any osteogenic-inducing chemical components or growth factors. The findings that osteogenic differentiation of hiPSC can be achieved through biomaterial-based cues alone present new avenues for personalized regenerative medicine. Such biomaterials that could not only act as structural scaffolds, but could also provide tissue-specific functions such as directing stem cell differentiation commitment, have great potential in bone tissue engineering.
Keywords: Bone tissue engineering; Calcium phosphate; Gelatin methacrylate; Human induced pluripotent stem cells; Osteogenic differentiation.
Copyright © 2014 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


References
-
- Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends in molecular medicine. 2009;15:59–68. - PubMed
-
- Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiological reviews. 2005;85:635–78. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. cell. 2007;131:861–72. - PubMed
-
- Teng S, Liu C, Krettek C, Jagodzinski M. The Application of Induced Pluripotent Stem Cells for Bone Regeneration: Current Progress and Prospects. Tissue Engineering Part B: Reviews. 2013 - PubMed
-
- Illich DJ, Demir N, Stojković M, Scheer M, Rothamel D, Neugebauer J, et al. Concise review: induced pluripotent stem cells and lineage reprogramming: prospects for bone regeneration. Stem Cells. 2011;29:555–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

