Catalytic enantioselective protoboration of disubstituted allenes. Access to alkenylboron compounds in high enantiomeric purity
- PMID: 25153792
- PMCID: PMC4156252
- DOI: 10.1021/ol5022417
Catalytic enantioselective protoboration of disubstituted allenes. Access to alkenylboron compounds in high enantiomeric purity
Abstract
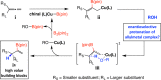
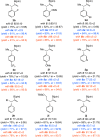
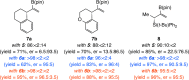
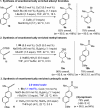
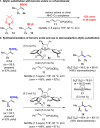
Proto-boryl additions to 1,1-disubstituted allenes in the presence of 1.0-5.0 mol % of chiral NHC-Cu complexes, B2(pin)2, and t-BuOH proceed to afford alkenyl-B(pin) products in up to 98% yield, >98:2 site selectivity, and 98:2 er. The enantiomerically enriched alkenylboron products can be converted to otherwise difficult-to-access alkenyl bromides, methyl ketones or carboxylic acids. What's more, the corresponding boronic acids may be used in highly stereoselective NHC-Cu-catalyzed allylic substitution reactions.
Figures
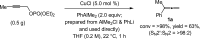

References
-
- Yus M.; Gonzalez-Gomez J. C.; Foubelo F. Chem. Rev. 2013, 113, 5595. - PubMed
-
-
For recent reviews on enantioselective protonations, see:
- Blanchet J.; Baudoux J.; Amere M.; Lasne M.-C.; Rouden J. Eur. J. Org. Chem. 2008, 5493.
- Mohr J. T.; Hong A. Y.; Stoltz B. M. Nat. Chem. 2009, 1, 359. - PMC - PubMed
-
For a recent relevant report, see:
- Cheon C. H.; Kanno O.; Toste F. D. J. Am. Chem. Soc. 2011, 133, 13248. - PMC - PubMed
-
-
- Lee Y.; Hoveyda A. H. J. Am. Chem. Soc. 2009, 131, 3160. - PMC - PubMed
- Lee Y.; Jang H.; Hoveyda A. H. J. Am. Chem. Soc. 2009, 131, 18234. - PMC - PubMed
- Coberán R.; Mszar N. M.; Hoveyda A. H. Angew. Chem., Int. Ed. 2011, 50, 7079. - PubMed
- Meng F.; Jang H.; Hoveyda A. H. Chem.—Eur. J. 2013, 19, 3204. - PubMed
-
See also:
- Matsuda N.; Hirano K.; Satoh T.; Miura M. J. Am. Chem. Soc. 2013, 135, 4934. - PubMed
- Meng F.; Haeffner J.; Hoveyda A. H. J. Am. Chem. Soc. 2014, 136, 11304. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

